Adipose tissue senescence is mediated by increased ATP content after a short-term high-fat diet exposure
- PMID: 34278707
- PMCID: PMC8373332
- DOI: 10.1111/acel.13421
Adipose tissue senescence is mediated by increased ATP content after a short-term high-fat diet exposure
Abstract
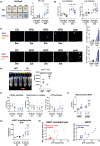
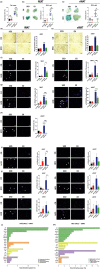
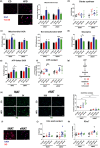
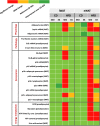
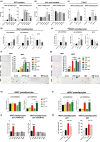
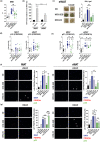
In the context of obesity, senescent cells accumulate in white adipose tissue (WAT). The cellular underpinnings of WAT senescence leading to insulin resistance are not fully elucidated. The objective of the current study was to evaluate the presence of WAT senescence early after initiation of high-fat diet (HFD, 1-10 weeks) in 5-month-old male C57BL/6J mice and the potential role of energy metabolism. We first showed that WAT senescence occurred 2 weeks after HFD as evidenced in whole WAT by increased senescence-associated ß-galactosidase activity and cyclin-dependent kinase inhibitor 1A and 2A expression. WAT senescence affected various WAT cell populations, including preadipocytes, adipose tissue progenitors, and immune cells, together with adipocytes. WAT senescence was associated with higher glycolytic and mitochondrial activity leading to enhanced ATP content in HFD-derived preadipocytes, as compared with chow diet-derived preadipocytes. One-month daily exercise, introduced 5 weeks after HFD, was an effective senostatic strategy, since it reversed WAT cellular senescence, while reducing glycolysis and production of ATP. Interestingly, the beneficial effect of exercise was independent of body weight and fat mass loss. We demonstrated that WAT cellular senescence is one of the earliest events occurring after HFD initiation and is intimately linked to the metabolic state of the cells. Our data uncover a critical role for HFD-induced elevated ATP as a local danger signal inducing WAT senescence. Exercise exerts beneficial effects on adipose tissue bioenergetics in obesity, reversing cellular senescence, and metabolic abnormalities.
Keywords: ATP; adipose tissue senescence; bioenergetics; exercise; obesity; xanthine oxidase.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
References
-
- Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Janich, P., Morton, J. P., Athineos, D., Kang, T.‐W., Lasitschka, F., Andrulis, M., Pascual, G., Morris, K. J., Khan, S., Jin, H., Dharmalingam, G., Snijders, A. P., Carroll, T., Capper, D., Pritchard, C., … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15, 978–990. 10.1038/ncb2784 - DOI - PMC - PubMed
-
- Anderson, R., Lagnado, A., Maggiorani, D., Walaszczyk, A., Dookun, E., Chapman, J., Birch, J., Salmonowicz, H., Ogrodnik, M., Jurk, D., Proctor, C., Correia‐Melo, C., Victorelli, S., Fielder, E., Berlinguer‐Palmini, R., Owens, A., Greaves, L. C., Kolsky, K. L., Parini, A., … Passos, J. F. (2019). Length‐independent telomere damage drives post‐mitotic cardiomyocyte senescence. The EMBO Journal, 38(5), 10.15252/embj.2018100492 - DOI - PMC - PubMed
-
- Baker, D. J., Childs, B. G., Durik, M., Wijers, M. E., Sieben, C. J., Zhong, J., A. Saltness, R., Jeganathan, K. B., Verzosa, G. C., Pezeshki, A., Khazaie, K., Miller, J. D., & van Deursen, J. M. (2016). Naturally occurring p16(Ink4a)‐positive cells shorten healthy lifespan. Nature, 530, 184–189. 10.1038/nature16932 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

