Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA
- PMID: 34278827
- PMCID: PMC8530153
- DOI: 10.2217/fon-2021-0549
Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA
Abstract
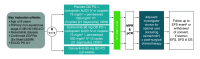
Osimertinib is a third-generation, irreversible oral EGFR-tyrosine kinase inhibitor), that potently inhibits EGFR-tyrosine kinase inhibitor-sensitizing mutations and T790M resistance mutations together with efficacy in CNS metastases in patients with non-small-cell lung cancer (NSCLC). Here we describe the rationale and design for the Phase III NeoADAURA study (NCT04351555), which will evaluate neoadjuvant osimertinib with or without chemotherapy versus chemotherapy alone prior to surgery, in patients with resectable stage II-IIIB N2 EGFR mutation-positive NSCLC. The primary end point is centrally assessed major pathological response at the time of resection. Secondary end points include event-free survival, pathological complete response, nodal downstaging at the time of surgery, disease-free survival, overall survival and health-related quality of life. Safety and tolerability will also be assessed. Trial Registration number: NCT04351555 (ClinicalTrials.gov).
Keywords: EGFR-TKI-sensitizing mutations; EGFR-tyrosine kinase inhibitor; neoadjuvant; non-small-cell lung cancer; osimertinib; resectable.
Plain language summary
Lay abstract A plain language version of this article is available and is published alongside the paper online: www.futuremedicine.com/doi/suppl/10.2217/fon-2021-0549.
Conflict of interest statement
This trial (NCT04351555) was funded by AstraZeneca, the manufacturer of osimertinib. The funder contributed to the conception and design of the trial; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication and as such are included in the author list and acknowledgments. M Tsuboi – advisory boards: AstraZeneca, MSD, Novartis; honoraria received from promotional activities: AstraZeneca KK, Bristol-Myers Squibb KK, Chugai Pharmaceutical Co Ltd, Eli Lilly Japan, Johnson & Johnson Japan, Medtronic Japan, Ono Pharmaceutical Co Ltd, Taiho Pharma, Teijin Pharma. W Weder – consultant: AstraZeneca, Medtronic. C Escriu – speakers bureau: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Pfizer; advisory boards: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, MSD. C Blakely – advisory board: Blueprint Medicines, Bayer; consultant: Amgen. J He – nothing to disclose. S Dacic – advisory board: AstraZeneca, Takeda, Janssen; consultant: AstraZeneca. Y Yatabe – advisory boards: AstraZeneca, Daiichi-Sankyo, MSD, Amgen, Takeda; honoraria received from promotional activities: Alilent/Dako, AstraZeneca, Chugai Pharma, MDS, Novartis, Pfizer, Roche/Ventana, Thermo Fisher Scientific. L Zeng – ownership or stock interests: AstraZeneca; employment: AstraZeneca. A Walding – ownership or stock interests: AstraZeneca; employment: AstraZeneca. JE Chaft – consultant: AstraZeneca, Flame Biosciences, Genentech, Merck, Novartis, Jannsen. Cancer Center Support Grant P30 CA00874. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors would like to acknowledge S Cotterill of Ashfield MedComms, an Ashfield Health company, and C McCleverty contracted to Ashfield MedComms, for medical writing support that was funded by AstraZeneca in accordance with Good Publications Practice (GPP3) guidelines (
Figures
References
-
- Le Chevalier T. Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann. Oncol. 21(Suppl. 7), vii196–198 (2010). - PubMed
-
- Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest 123(6), 2096–2103 (2003). - PubMed
-
- Cagle PT, Allen TC, Olsen RJ. Lung cancer biomarkers: present status and future developments. Arch. Pathol. Lab. Med. 137(9), 1191–1198 (2013). - PubMed
-
- Postmus PE, Kerr KM, Oudkerk M et al. Early-stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 28(Suppl. 4), iv1–iv21 (2017). - PubMed
-
- Goldstraw P, Chansky K, Crowley J et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 11(1), 39–51 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
