Dual-Responsive Material Based on Catechol-Modified Self-Immolative Poly(Disulfide) Backbones
- PMID: 34279056
- PMCID: PMC8518080
- DOI: 10.1002/anie.202108698
Dual-Responsive Material Based on Catechol-Modified Self-Immolative Poly(Disulfide) Backbones
Abstract
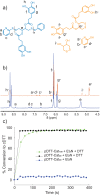
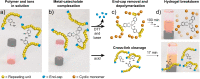
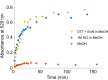
Functional materials engineered to degrade upon triggering are in high demand due their potentially lower impact on the environment as well as their use in sensing and in medical applications. Here, stimuli-responsive polymers are prepared by decorating a self-immolative poly(dithiothreitol) backbone with pendant catechol units. The highly functional polymer is fashioned into stimuli-responsive gels, formed through pH-dependent catecholato-metal ion cross-links. The gels degrade in response to specific environmental changes, either by addressing the pH responsive, non-covalent, catecholato-metal complexes, or by addition of a thiol. The latter stimulus triggers end-to-end depolymerization of the entire self-immolative backbone through end-cap replacement via thiol-disufide exchanges. Gel degradation is visualized by release of a dye from the supramolecular gel as it itself is converted into smaller molecules.
Keywords: catechol; hydrogel; polymers; self-immolative; stimuli-responsive.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nguyen Q. V., Huynh D. P., Park J. H., Lee D. S., Eur. Polym. J. 2015, 72, 602–619.
-
- Shi Q., Liu H., Tang D. D., Li Y. H., Li X. J., Xu F., NPG Asia Mater. 2019, 11, 64.
-
- Zhang D., Ren B., Zhang Y., Xu L., Huang Q., He Y., Li X., Wu J., Yang J., Chen Q., Chang Y., Zheng J., J. Mater. Chem. B 2020, 8, 3171–3191; - PubMed
- Xia S., Zhang Q., Song S. X., Duan L. J., Gao G. H., Chem. Mater. 2019, 31, 9522–9531.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

