A Molecular Simulation Study of Silica/Polysulfone Mixed Matrix Membrane for Mixed Gas Separation
- PMID: 34279343
- PMCID: PMC8271399
- DOI: 10.3390/polym13132199
A Molecular Simulation Study of Silica/Polysulfone Mixed Matrix Membrane for Mixed Gas Separation
Abstract
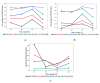
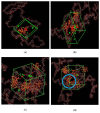
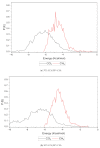
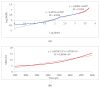
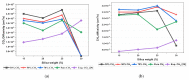
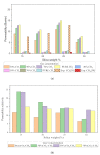
Polysulfone-based mixed matrix membranes (MMMs) incorporated with silica nanoparticles are a new generation material under ongoing research and development for gas separation. However, the attributes of a better-performing MMM cannot be precisely studied under experimental conditions. Thus, it requires an atomistic scale study to elucidate the separation performance of silica/polysulfone MMMs. As most of the research work and empirical models for gas transport properties have been limited to pure gas, a computational framework for molecular simulation is required to study the mixed gas transport properties in silica/polysulfone MMMs to reflect real membrane separation. In this work, Monte Carlo (MC) and molecular dynamics (MD) simulations were employed to study the solubility and diffusivity of CO2/CH4 with varying gas concentrations (i.e., 30% CO2/CH4, 50% CO2/CH4, and 70% CO2/CH4) and silica content (i.e., 15-30 wt.%). The accuracy of the simulated structures was validated with published literature, followed by the study of the gas transport properties at 308.15 K and 1 atm. Simulation results concluded an increase in the free volume with an increasing weight percentage of silica. It was also found that pure gas consistently exhibited higher gas transport properties when compared to mixed gas conditions. The results also showed a competitive gas transport performance for mixed gases, which is more apparent when CO2 increases. In this context, an increment in the permeation was observed for mixed gas with increasing gas concentrations (i.e., 70% CO2/CH4 > 50% CO2/CH4 > 30% CO2/CH4). The diffusivity, solubility, and permeability of the mixed gases were consistently increasing until 25 wt.%, followed by a decrease for 30 wt.% of silica. An empirical model based on a parallel resistance approach was developed by incorporating mathematical formulations for solubility and permeability. The model results were compared with simulation results to quantify the effect of mixed gas transport, which showed an 18% and 15% percentage error for the permeability and solubility, respectively, in comparison to the simulation data. This study provides a basis for future understanding of MMMs using molecular simulations and modeling techniques for mixed gas conditions that demonstrate real membrane separation.
Keywords: CO2/CH4 gas transport; empirical modelling; mixed gas; mixed matrix membrane; molecular simulation; polysulfone; silica.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
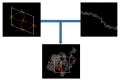
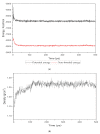
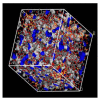

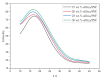




References
-
- Sellaro M., Brunetti A., Drioli E., Barbieri G. CO2-CH4 Membrane separation. Chim. Ambiente. 2015 doi: 10.17374/CI.2015.97.5.14. - DOI
-
- Zhang Y., Sunarso J., Liu S., Wang R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas. Control. 2013;12:84–107. doi: 10.1016/j.ijggc.2012.10.009. - DOI
-
- Baker R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002;41:1393–1411. doi: 10.1021/ie0108088. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

