Assessment of the Efficacy of Olive Leaf (Olea europaea L.) Extracts in the Treatment of Colorectal Cancer and Prostate Cancer Using In Vitro Cell Models
- PMID: 34279409
- PMCID: PMC8272070
- DOI: 10.3390/molecules26134069
Assessment of the Efficacy of Olive Leaf (Olea europaea L.) Extracts in the Treatment of Colorectal Cancer and Prostate Cancer Using In Vitro Cell Models
Abstract
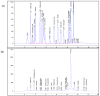
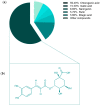
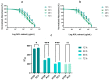
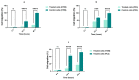
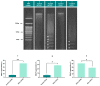
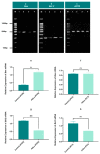
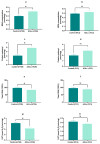
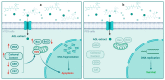
Cancer is one of the most serious public health issues worldwide, ranking second only to cardiovascular diseases as a cause of death. Numerous plant extracts have extraordinary health benefits and have been used for centuries to treat a variety of ailments with few side effects. Olive leaves have a long history of medicinal and therapeutic use. In this study, the anti-cancer properties of an olive leaf extract were investigated in vitro using colorectal and prostate cancer cell lines (HT29 and PC3, respectively). A high-performance liquid chromatography analysis showed that the olive leaf extract contained a high chlorogenic acid content. Accordingly, chlorogenic acid may be related to the observed effects of the aqueous extract on cancer cells, including increased inhibition of cancer cell growth, migration, DNA fragmentation, cell cycle arrest at the S phase, reactive oxygen species (ROS) production, and altered gene expression. The effects of the extracts were greater in HT29 than in PC3 cells. These results suggest that chlorogenic acid, the main constituent in the olive extract, is a promising new anti-cancer agent. Further analyses should focus on its in vivo effects on colorectal tumor models, both alone and in combination with established agents.
Keywords: chlorogenic acid; colorectal cancer; olive leaf extract; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

