Chlamydia Spreads to the Large Intestine Lumen via Multiple Pathways
- PMID: 34280037
- PMCID: PMC8445164
- DOI: 10.1128/IAI.00254-21
Chlamydia Spreads to the Large Intestine Lumen via Multiple Pathways
Abstract
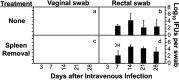
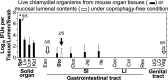
Chlamydia in the genital tract is known to spread via the blood circulation system to the large intestine lumen to achieve long-lasting colonization. However, the precise pathways by which genital Chlamydia accesses the large intestine lumen remain unclear. The spleen was recently reported to be critical for chlamydial spreading. In the current study, it was found that following intravaginal inoculation with Chlamydia, mice with and without splenectomy both yielded infectious Chlamydia on rectal swabs, indicating that the spleen is not essential for genital Chlamydia to spread to the gastrointestinal tract. This conclusion was validated by the observation that intravenously inoculated Chlamydia was also detected on the rectal swabs of mice regardless of splenectomy. Careful comparison of the tissue distribution of live chlamydial organisms following intravenous inoculation revealed redundant pathways by which Chlamydia can reach the large intestine lumen. The intravenously inoculated Chlamydia was predominantly recruited to the spleen within 12 h and then detected in the stomach lumen by 24 h, in the intestinal lumen by 48 h, and on rectal swabs by 72 h. These observations suggest a potential spleen-to-stomach pathway for hematogenous Chlamydia to reach the large intestine lumen. This conclusion was supported by the observation made in mice under coprophagy-free condition. However, in the absence of spleen, hematogenous Chlamydia was predominantly recruited to the liver and then simultaneously detected in the intestinal tissue and lumen, suggesting a potential liver-to-intestine pathway for Chlamydia to reach the large intestine lumen. Thus, genital/hematogenous Chlamydia may reach the large intestine lumen via multiple redundant pathways.
Keywords: Chlamydia; GI tract; liver to intestine; spleen to stomach; spreading pathways.
Figures



Similar articles
-
Antigen-Specific CD4+ T Cell-Derived Gamma Interferon Is Both Necessary and Sufficient for Clearing Chlamydia from the Small Intestine but Not the Large Intestine.Infect Immun. 2019 May 21;87(6):e00055-19. doi: 10.1128/IAI.00055-19. Print 2019 Jun. Infect Immun. 2019. PMID: 30962403 Free PMC article.
-
Chlamydia Deficient in Plasmid-Encoded pGP3 Is Prevented from Spreading to Large Intestine.Infect Immun. 2020 May 20;88(6):e00120-20. doi: 10.1128/IAI.00120-20. Print 2020 May 20. Infect Immun. 2020. PMID: 32205401 Free PMC article.
-
Gastrointestinal Chlamydia-Induced CD8+ T Cells Promote Chlamydial Pathogenicity in the Female Upper Genital Tract.Infect Immun. 2021 Sep 16;89(10):e0020521. doi: 10.1128/IAI.00205-21. Epub 2021 Jul 6. Infect Immun. 2021. PMID: 34227838 Free PMC article.
-
Chlamydia overcomes multiple gastrointestinal barriers to achieve long-lasting colonization.Trends Microbiol. 2021 Nov;29(11):1004-1012. doi: 10.1016/j.tim.2021.03.011. Epub 2021 Apr 14. Trends Microbiol. 2021. PMID: 33865675 Free PMC article. Review.
-
Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract - A Two-Hit Hypothesis.Trends Microbiol. 2018 Jul;26(7):611-623. doi: 10.1016/j.tim.2017.12.002. Epub 2017 Dec 27. Trends Microbiol. 2018. PMID: 29289422 Free PMC article. Review.
Cited by
-
Diverse animal models for Chlamydia infections: unraveling pathogenesis through the genital and gastrointestinal tracts.Front Microbiol. 2024 Mar 28;15:1386343. doi: 10.3389/fmicb.2024.1386343. eCollection 2024. Front Microbiol. 2024. PMID: 38605708 Free PMC article. Review.
-
Induction of Transmucosal Protection by Oral Vaccination with an Attenuated Chlamydia.Infect Immun. 2023 May 16;91(5):e0004323. doi: 10.1128/iai.00043-23. Epub 2023 Apr 10. Infect Immun. 2023. PMID: 37036335 Free PMC article.
-
Chlamydia muridarum Causes Persistent Subclinical Infection and Elicits Innate and Adaptive Immune Responses in C57BL/6J, BALB/cJ and J:ARC(S) Mice Following Exposure to Shedding Mice.bioRxiv [Preprint]. 2024 Sep 6:2024.07.16.603732. doi: 10.1101/2024.07.16.603732. bioRxiv. 2024. Update in: Comp Med. 2024 Dec 01;74(6):373-391. doi: 10.30802/AALAS-CM-24-057. PMID: 39071441 Free PMC article. Updated. Preprint.
-
Chlamydia muridarum Causes Persistent Subclinical Infection and Elicits Innate and Adaptive Immune Responses in C57BL/6J, BALB/cJ, and J:ARC(S) Mice Following Exposure to Shedding Mice.Comp Med. 2024 Dec 1;74(6):373-391. doi: 10.30802/AALAS-CM-24-057. Comp Med. 2024. PMID: 39853328 Free PMC article.
-
Regulation of chlamydial colonization by IFNγ delivered via distinct cells.Trends Microbiol. 2023 Mar;31(3):270-279. doi: 10.1016/j.tim.2022.09.002. Epub 2022 Sep 26. Trends Microbiol. 2023. PMID: 36175276 Free PMC article. Review.
References
-
- Murthy AK, Li W, Guentzel MN, Zhong G, Arulanandam BP. 2011. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine 29:2519–2522. doi:10.1016/j.vaccine.2011.01.074. - DOI - PMC - PubMed
-
- Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi:10.1371/journal.pone.0095076. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

