Participating in a vaccine trial for COVID-19 in Senegal: trust and information
- PMID: 34280070
- PMCID: PMC8828143
- DOI: 10.1080/21645515.2021.1951097
Participating in a vaccine trial for COVID-19 in Senegal: trust and information
Abstract
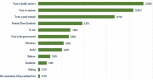
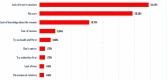
This research aims to understand the level and determinants of people's willingness to participate in a vaccine trial for COVID-19 in Senegal. We conducted a telephone survey among a marginal quota sample of 607 people over 18 years of age. Only 44.3% of the participants wanted to participate in a vaccine trial for COVID-19, with females intending to participate more than males (AOR = 1.82, 95% CI [1.22-2.72]). Participants who intended to be vaccinated against COVID-19 (AOR = 6.48, 95% CI [4.12-10.4]) and who thought that being infected with the coronavirus would have a significant impact on their health (AOR = 2.34, 95% CI [1.57, 3.51]) were more likely to agree to take part in the COVID-19 vaccine trial. Confidence in the vaccine, health personnel, and the government in the fight against the pandemic are key factors in participants' willingness to participate in a vaccine trial in Senegal.
Keywords: COVID-19; Senegal; Vaccine trial; acceptability; participation.
Figures
Similar articles
-
The relationship between trust in federal oversight of vaccine safety and willingness to participate in COVID-19 clinical trials: a repeated measures study of Philadelphia residents (September 2021 - March 2023).BMC Public Health. 2024 Jul 31;24(1):2059. doi: 10.1186/s12889-024-19602-7. BMC Public Health. 2024. PMID: 39085794 Free PMC article.
-
Trends in willingness to receive COVID-19 vaccines among healthcare workers in India: Findings from repeated cross-sectional national surveys.Front Public Health. 2022 Oct 3;10:994206. doi: 10.3389/fpubh.2022.994206. eCollection 2022. Front Public Health. 2022. PMID: 36262227 Free PMC article.
-
The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Sep 6;22(1):592. doi: 10.1186/s13063-021-05484-2. Trials. 2021. PMID: 34488843 Free PMC article.
-
Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic.Vaccine. 2020 Oct 21;38(45):7002-7006. doi: 10.1016/j.vaccine.2020.09.041. Epub 2020 Sep 17. Vaccine. 2020. PMID: 32988688 Free PMC article.
-
Intention to participate in COVID-19 vaccine clinical trials in May 2021: a cross-sectional survey in the general French population.Hum Vaccin Immunother. 2022 Nov 30;18(5):2072630. doi: 10.1080/21645515.2022.2072630. Epub 2022 May 13. Hum Vaccin Immunother. 2022. PMID: 35561252 Free PMC article.
Cited by
-
Vaccine inequity: a threat to Africa's recovery from COVID-19.Trop Med Health. 2023 Dec 19;51(1):69. doi: 10.1186/s41182-023-00564-2. Trop Med Health. 2023. PMID: 38111032 Free PMC article. Review.
-
Management of the COVID crisis in Reunion Island (SW Indian Ocean): representations of COVID-19 and acceptance of public health measures.Health Psychol Behav Med. 2023 Sep 1;11(1):2252902. doi: 10.1080/21642850.2023.2252902. eCollection 2023. Health Psychol Behav Med. 2023. PMID: 37674594 Free PMC article.
-
Calculation, knowledge, and identity: Dimensions of trust when making COVID-19 vaccination choices in China.SSM Qual Res Health. 2023 Dec;4:100288. doi: 10.1016/j.ssmqr.2023.100288. Epub 2023 Jun 1. SSM Qual Res Health. 2023. PMID: 37334196 Free PMC article.
-
Factors associated with COVID-19 vaccine intention in Benin in 2021: A cross-sectional study.Vaccine X. 2022 Dec;12:100237. doi: 10.1016/j.jvacx.2022.100237. Epub 2022 Nov 3. Vaccine X. 2022. PMID: 36348760 Free PMC article.
-
Use of interviewer-administered telephone surveys during infectious disease outbreaks, epidemics and pandemics: a scoping review.BMJ Glob Health. 2023 May;8(5):e011109. doi: 10.1136/bmjgh-2022-011109. BMJ Glob Health. 2023. PMID: 37137536 Free PMC article.
References
-
- Lachenal G. Le médicament qui devait sauver l’Afrique: un scandale pharmaceutique aux colonies. Paris (France): La Découverte; 2014.
-
- Moulin AM, Chabrol F, Ouvrier A. Chapitre 24. Histoire d’un vaccin pas comme les autres : les premiers pas du vaccin contre l’hépatite B au Sénégal. In: Delaunay V, Desclaux A, Sokhna C, editors. Niakhar, mémoires et perspectives. Montpellier (France): IRD Éditions;2018. p. 489–510. doi:10.4000/books.irdeditions.31872. - DOI
-
- Ouvrier A. La recherche médicale en Afrique est un moyen pour l’Occident de tester des médicaments dangereux. Des idées reçues en santé mondiale. Montréal (Canada): Presses de l’Université de Montréal. p. 215–18; 2015. doi:10.4000/books.pum.3607 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical