Immune selection suppresses the emergence of drug resistance in malaria parasites but facilitates its spread
- PMID: 34280179
- PMCID: PMC8321109
- DOI: 10.1371/journal.pcbi.1008577
Immune selection suppresses the emergence of drug resistance in malaria parasites but facilitates its spread
Abstract
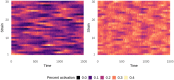
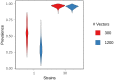
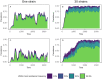
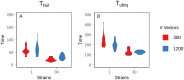
Although drug resistance in Plasmodium falciparum typically evolves in regions of low transmission, resistance spreads readily following introduction to regions with a heavier disease burden. This suggests that the origin and the spread of resistance are governed by different processes, and that high transmission intensity specifically impedes the origin. Factors associated with high transmission, such as highly immune hosts and competition within genetically diverse infections, are associated with suppression of resistant lineages within hosts. However, interactions between these factors have rarely been investigated and the specific relationship between adaptive immunity and selection for resistance has not been explored. Here, we developed a multiscale, agent-based model of Plasmodium parasites, hosts, and vectors to examine how host and parasite dynamics shape the evolution of resistance in populations with different transmission intensities. We found that selection for antigenic novelty ("immune selection") suppressed the evolution of resistance in high transmission settings. We show that high levels of population immunity increased the strength of immune selection relative to selection for resistance. As a result, immune selection delayed the evolution of resistance in high transmission populations by allowing novel, sensitive lineages to remain in circulation at the expense of the spread of a resistant lineage. In contrast, in low transmission settings, we observed that resistant strains were able to sweep to high population prevalence without interference. Additionally, we found that the relationship between immune selection and resistance changed when resistance was widespread. Once resistance was common enough to be found on many antigenic backgrounds, immune selection stably maintained resistant parasites in the population by allowing them to proliferate, even in untreated hosts, when resistance was linked to a novel epitope. Our results suggest that immune selection plays a role in the global pattern of resistance evolution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Malaria Report. World Health Organization. 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

