Trends in Accuracy and Comprehensiveness of Pathology Reports for Resected NSCLC in a High Mortality Area of the United States
- PMID: 34280563
- PMCID: PMC9039869
- DOI: 10.1016/j.jtho.2021.06.027
Trends in Accuracy and Comprehensiveness of Pathology Reports for Resected NSCLC in a High Mortality Area of the United States
Abstract
Introduction: Complete and accurate pathology reports are vital to postoperative prognostication and management. We evaluated the impact of three interventions across a diverse group of hospitals on pathology reports of postresection NSCLC.
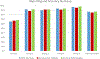
Methods: We evaluated pathology reports for patients who underwent curative-intent surgical resection for NSCLC, at 11 institutions within four contiguous Dartmouth Hospital Referral Regions in Arkansas, Mississippi, and Tennessee from 2004 to 2020, for completeness and accuracy, before and after the following three quality improvement interventions: education (feedback to heighten awareness); synoptic reporting; and a lymph node specimen collection kit. We compared the proportion of pathology reports with the six most important items for postoperative management (specimen type, tumor size, histologic type, pathologic [p] T-category, pN-category, margin status) across the following six patient cohorts: preintervention control, postintervention with four different combinations of interventions, and a contemporaneous nonintervention external control.
Results: In the postintervention era, the odds of reporting all key items were eight times higher than those in the preintervention era (OR = 8.3, 95 % confidence interval [CI]: 6.7-10.2, p < 0.0001). There were sixfold and eightfold increases in the odds of accurate pT- and pN-category reporting in the postintervention era compared with the preintervention era (pT OR = 5.7, 95 % CI: 4.7-6.9; pN OR = 8.0, 95 % CI: 6.5-10.0, both p < 0.0001). Within the intervention groups, the odds of reporting all six key items, accurate pT category, and accurate pN-category were highest in patients who received all three interventions.
Conclusions: Gaps in the quality of NSCLC pathologic reportage can be identified, quantified, and corrected by rationally designed interventions.
Keywords: Lymph node kit; NSCLC; Quality improvement; Synoptic reporting.
Copyright © 2021 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The remaining authors declare no conflict of interest.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. - PubMed
-
- Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline update. J Clin Oncol. 2017;35:2960–2974. - PubMed
-
- Zarbo RJ. Interinstitutional assessment of colorectal carcinoma surgical pathology report adequacy. A College of American Pathologists Q-Probes study of practice patterns from 532 laboratories and 15,940 reports. Arch Pathol Lab Med. 1992;116:1113–1119. - PubMed
-
- Idowu MO, Bekeris LG, Raab S, Ruby SG, Nakhleh RE. Adequacy of surgical pathology reporting of cancer: a College of American Pathologists Q-Probes study of 86 institutions. Arch Pathol Lab Med. 2010;134:969–974. - PubMed
-
- Gal AA, Marchevsky AM, Travis WD, Cancer Committee, College of American Pathologists. Updated protocol for the examination of specimens from patients with carcinoma of the lung. Arch Pathol Lab Med. 2003;127:1304–1313. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

