Possible Link between SARS-CoV-2 Infection and Parkinson's Disease: The Role of Toll-Like Receptor 4
- PMID: 34281186
- PMCID: PMC8269350
- DOI: 10.3390/ijms22137135
Possible Link between SARS-CoV-2 Infection and Parkinson's Disease: The Role of Toll-Like Receptor 4
Abstract
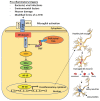
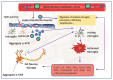
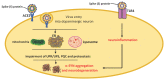
Parkinson's disease (PD) is the most common neurodegenerative motor disorder characterized by selective degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the midbrain, depletion of dopamine (DA), and impaired nigrostriatal pathway. The pathological hallmark of PD includes the aggregation and accumulation α-synuclein (α-SYN). Although the precise mechanisms underlying the pathogenesis of PD are still unknown, the activation of toll-like receptors (TLRs), mainly TLR4 and subsequent neuroinflammatory immune response, seem to play a significant role. Mounting evidence suggests that viral infection can concur with the precipitation of PD or parkinsonism. The recently identified coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of ongoing pandemic coronavirus disease 2019 (COVID-19), responsible for 160 million cases that led to the death of more than three million individuals worldwide. Studies have reported that many patients with COVID-19 display several neurological manifestations, including acute cerebrovascular diseases, conscious disturbance, and typical motor and non-motor symptoms accompanying PD. In this review, the neurotropic potential of SARS-CoV-2 and its possible involvement in the pathogenesis of PD are discussed. Specifically, the involvement of the TLR4 signaling pathway in mediating the virus entry, as well as the massive immune and inflammatory response in COVID-19 patients is explored. The binding of SARS-CoV-2 spike (S) protein to TLR4 and the possible interaction between SARS-CoV-2 and α-SYN as contributing factors to neuronal death are also considered.
Keywords: COVID-19; Parkinson’s disease; SARS-CoV-2; neuroinflammation; synuclein; toll-like receptor 4.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

