Upregulated Chemokine and Rho-GTPase Genes Define Immune Cell Emigration into Salivary Glands of Sjögren's Syndrome-Susceptible C57BL/6.NOD- Aec1Aec2 Mice
- PMID: 34281229
- PMCID: PMC8267620
- DOI: 10.3390/ijms22137176
Upregulated Chemokine and Rho-GTPase Genes Define Immune Cell Emigration into Salivary Glands of Sjögren's Syndrome-Susceptible C57BL/6.NOD- Aec1Aec2 Mice
Abstract
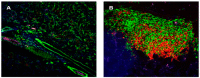
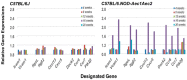
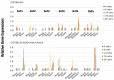
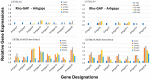
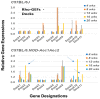
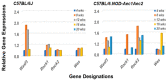
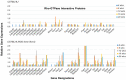
The C57BL/6.NOD-Aec1Aec2 mouse is considered a highly appropriate model of Sjögren's Syndrome (SS), a human systemic autoimmune disease characterized primarily as the loss of lacrimal and salivary gland functions. This mouse model, as well as other mouse models of SS, have shown that B lymphocytes are essential for the development and onset of observed clinical manifestations. More recently, studies carried out in the C57BL/6.IL14α transgenic mouse have indicated that the marginal zone B (MZB) cell population is responsible for development of SS disease, reflecting recent observations that MZB cells are present in the salivary glands of SS patients and most likely initiate the subsequent loss of exocrine functions. Although MZB cells are difficult to study in vivo and in vitro, we have carried out an ex vivo investigation that uses temporal global RNA transcriptomic analyses to profile differentially expressed genes known to be associated with cell migration. Results indicate a temporal upregulation of specific chemokine, chemokine receptor, and Rho-GTPase genes in the salivary glands of C57BL/6.NOD-Aec1Aec2 mice that correlate with the early appearance of periductal lymphocyte infiltrations. Using the power of transcriptomic analyses to better define the genetic profile of lymphocytic emigration into the salivary glands of SS mice, new insights into the underlying mechanisms of SS disease development and onset begin to come into focus, thereby establishing a foundation for further in-depth and novel investigations of the covert and early overt phases of SS disease at the cellular level.
Keywords: C57BL/6.NOD-Aec1Aec2 mice; DOCK molecules; GTP-GAP; GTP-GEF; RNA transcriptome microarray; Rho-GTPases; Sjögren’s syndrome; marginal zone B cells.
Conflict of interest statement
The authors declare no conflict of interest, and the NIH had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, nor in the decision to publish the results.
Figures
Similar articles
-
Marginal Zone B (MZB) Cells: Comparison of the Initial Identification of Immune Activity Leading to Dacryoadenitis and Sialadenitis in Experimental Sjögren's Syndrome.Int J Mol Sci. 2023 Jul 30;24(15):12209. doi: 10.3390/ijms241512209. Int J Mol Sci. 2023. PMID: 37569583 Free PMC article.
-
Early Covert Appearance of Marginal Zone B Cells in Salivary Glands of Sjögren's Syndrome-Susceptible Mice: Initiators of Subsequent Overt Clinical Disease.Int J Mol Sci. 2021 Feb 15;22(4):1919. doi: 10.3390/ijms22041919. Int J Mol Sci. 2021. PMID: 33671965 Free PMC article.
-
A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren's Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses.Int J Mol Sci. 2022 May 29;23(11):6106. doi: 10.3390/ijms23116106. Int J Mol Sci. 2022. PMID: 35682784 Free PMC article.
-
New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model.Arch Oral Biol. 1999 May;44 Suppl 1:S21-5. doi: 10.1016/s0003-9969(99)00045-x. Arch Oral Biol. 1999. PMID: 10414851 Review.
-
Does HTLV-1 Infection Show Phenotypes Found in Sjögren's Syndrome?Viruses. 2022 Jan 6;14(1):100. doi: 10.3390/v14010100. Viruses. 2022. PMID: 35062304 Free PMC article. Review.
Cited by
-
Marginal Zone B (MZB) Cells: Comparison of the Initial Identification of Immune Activity Leading to Dacryoadenitis and Sialadenitis in Experimental Sjögren's Syndrome.Int J Mol Sci. 2023 Jul 30;24(15):12209. doi: 10.3390/ijms241512209. Int J Mol Sci. 2023. PMID: 37569583 Free PMC article.
-
Advances in Pathogenesis of Sjögren's Syndrome.J Immunol Res. 2021 Oct 7;2021:5928232. doi: 10.1155/2021/5928232. eCollection 2021. J Immunol Res. 2021. PMID: 34660815 Free PMC article.
-
Single-Cell Transcriptomics Reveals a Pivotal Role of DOCK2 in Sjögren Disease.ACR Open Rheumatol. 2024 Dec;6(12):927-943. doi: 10.1002/acr2.11738. Epub 2024 Oct 9. ACR Open Rheumatol. 2024. PMID: 39382155 Free PMC article.
-
Lessons from Animal Models in Sjögren's Syndrome.Int J Mol Sci. 2023 Aug 20;24(16):12995. doi: 10.3390/ijms241612995. Int J Mol Sci. 2023. PMID: 37629175 Free PMC article. Review.
-
Vasoactive intestinal peptide exerts therapeutic action by regulating PTEN in a model of Sjögren's disease.Immun Inflamm Dis. 2023 Jul;11(7):e936. doi: 10.1002/iid3.936. Immun Inflamm Dis. 2023. PMID: 37506142 Free PMC article.
References
-
- Vivino F.B., Bunya V.Y., Massaro-Giordano G., Johr C.R., Giattino S.L., Schorpion A., Shafer B., Peck A., Sivils K., Rasmussen A., et al. Sjogren’s Syndrome: An Update on Disease Pathogenesis, Clinical Manifestations and Treatment. Clin. Immunol. 2019;203:81–121. doi: 10.1016/j.clim.2019.04.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

