Roles of MicroRNAs in Osteogenesis or Adipogenesis Differentiation of Bone Marrow Stromal Progenitor Cells
- PMID: 34281266
- PMCID: PMC8269269
- DOI: 10.3390/ijms22137210
Roles of MicroRNAs in Osteogenesis or Adipogenesis Differentiation of Bone Marrow Stromal Progenitor Cells
Abstract
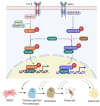
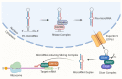
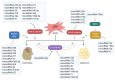
Bone marrow stromal cells (BMSCs) are multipotent cells which can differentiate into chondrocytes, osteoblasts, and fat cells. Under pathological stress, reduced bone formation in favour of fat formation in the bone marrow has been observed through a switch in the differentiation of BMSCs. The bone/fat switch causes bone growth defects and disordered bone metabolism in bone marrow, for which the mechanisms remain unclear, and treatments are lacking. Studies suggest that small non-coding RNAs (microRNAs) could participate in regulating BMSC differentiation by disrupting the post-transcription of target genes, leading to bone/fat formation changes. This review presents an emerging concept of microRNA regulation in the bone/fat formation switch in bone marrow, the evidence for which is assembled mainly from in vivo and in vitro human or animal models. Characterization of changes to microRNAs reveals novel networks that mediate signalling and factors in regulating bone/fat switch and homeostasis. Recent advances in our understanding of microRNAs in their control in BMSC differentiation have provided valuable insights into underlying mechanisms and may have significant potential in development of new therapeutics.
Keywords: BMSC differentiation; bone/fat formation; microRNAs.
Conflict of interest statement
The Authors declare that they have no conflict of interest.
Figures

References
-
- Bonfield T.L., Caplan A.I. Adult mesenchymal stem cells: An innovative therapeutic for lung diseases. Discov. Med. 2010;9:337–345. - PubMed
-
- Li J., Zuo B., Zhang L., Dai L., Zhang X. Osteoblast versus Adipocyte: Bone Marrow Microenvironment-Guided Epigenetic Control. Case Rep. Orthop. Res. 2018;1:2–18. doi: 10.1159/000489053. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

