Less Carcinogenic Chlorinated Estrogens Applicable to Hormone Replacement Therapy
- PMID: 34281275
- PMCID: PMC8268473
- DOI: 10.3390/ijms22137222
Less Carcinogenic Chlorinated Estrogens Applicable to Hormone Replacement Therapy
Abstract
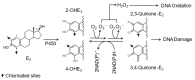
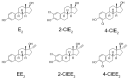
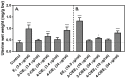
Human estrogens prescribed for hormone replacement therapy (HRT) are known to be potent carcinogens. To find safer estrogens, several chlorinated estrogens were synthesized and their carcinogenic potential were determined. A pellet containing either 2-chloro-17β-estradiol (2-ClE2) or 4-chloro-17β-estradiol (4-ClE2) was implanted subcutaneously for 52 weeks into August Copenhagen Irish (ACI) rats, a preferred animal model for human breast cancer. 17β-Estradiol (E2) frequently induced mammary tumors while both 2-ClE2 and 4-ClE2 did not. Their 17α-ethinyl forms, thought to be orally active estrogens, were also synthesized. Neither 2-chloro-17α-ethinylestradiol (2-ClEE2) nor 4-chloro-17α-ethinylestradiol (4-ClEE2) induced tumors. The less carcinogenic effects were supported by histological examination of mammary glands of ACI rats treated with the chlorinated estrogens. A chlorine atom positioned at the 2- or 4-position of E2 may prevent the metabolic activation, resulting in reducing the carcinogenicity. 2-ClE2 and 4-ClE2 administered subcutaneously and 2-ClEE2 and 4-ClEE2 given orally to ovariectomized rats all showed uterotrophic potency, albeit slightly weaker than that of E2. Our results indicate that less carcinogenic chlorinated estrogens retaining estrogenic potential could be safer alternatives to the carcinogenic estrogens now in use for HRT.
Keywords: DNA damage; chlorination; estrogen; hormone replacement therapy; mammary tumor; uterotrophic activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Carcinogenic potential of fluorinated estrogens in mammary tumorigenesis.Toxicol Lett. 2020 Jan;318:99-103. doi: 10.1016/j.toxlet.2019.10.015. Epub 2019 Oct 24. Toxicol Lett. 2020. PMID: 31669098
-
The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17beta-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16alpha-hydroxyestradiol, and 4-hydroxyestrone.J Endocrinol. 2004 Oct;183(1):91-9. doi: 10.1677/joe.1.05802. J Endocrinol. 2004. PMID: 15525577
-
Naturally-occurring estradiol-17beta-fatty acid esters, but not estradiol-17beta, preferentially induce mammary tumorigenesis in female rats: implications for an important role in human breast cancer.Toxicol Appl Pharmacol. 2008 Jun 15;229(3):332-41. doi: 10.1016/j.taap.2008.01.042. Epub 2008 Feb 20. Toxicol Appl Pharmacol. 2008. PMID: 18394671
-
Role of hormones in mammary cancer initiation and progression.J Mammary Gland Biol Neoplasia. 1998 Jan;3(1):49-61. doi: 10.1023/a:1018770218022. J Mammary Gland Biol Neoplasia. 1998. PMID: 10819504 Review.
-
Estrogenic treatment and liver functions.Ceska Gynekol. 2024;89(6):501-503. doi: 10.48095/cccg2024501. Ceska Gynekol. 2024. PMID: 39800550 Review. English.
References
-
- Colditz G.A., Hankinson S.E., Hunter D.J., Willett W.C., Manson J.E., Stampfer M.J., Hennekens C.H., Rosner B., Speizer F.E. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N. Engl. J. Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

