Evaluation of the AV7909 Anthrax Vaccine Toxicity in Sprague Dawley Rats Following Three Intramuscular Administrations
- PMID: 34281421
- PMCID: PMC8532110
- DOI: 10.1177/10915818211031239
Evaluation of the AV7909 Anthrax Vaccine Toxicity in Sprague Dawley Rats Following Three Intramuscular Administrations
Abstract
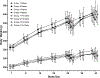
AV7909 is a next-generation anthrax vaccine under development for post-exposure prophylaxis following suspected or confirmed Bacillus anthracis exposure, when administered in conjunction with the recommended antibacterial regimen. AV7909 consists of the FDA-approved BioThrax® vaccine (anthrax vaccine adsorbed) and an immunostimulatory Toll-like receptor 9 agonist oligodeoxynucleotide adjuvant, CPG 7909. The purpose of this study was to evaluate the potential systemic and local toxicity of AV7909 when administered via repeat intramuscular injection to the right thigh muscle (biceps femoris) to male and female Sprague Dawley rats. The vaccine was administered on Days 1, 15, and 29 and the animals were assessed for treatment-related effects followed by a 2-week recovery period to evaluate the persistence or reversibility of any toxic effects. The AV7909 vaccine produced no apparent systemic toxicity based on evaluation of clinical observations, body weights, body temperature, clinical pathology, and anatomic pathology. Necrosis and inflammation were observed at the injection sites as well as in regional lymph nodes and adjacent tissues and were consistent with immune stimulation. Antibodies against B. anthracis protective antigen (PA) were detected in rats treated with the AV7909 vaccine, confirming relevance of this animal model for the assessment of systemic toxicity of AV7909. In contrast, sera of rats that received saline or soluble CPG 7909 alone were negative for anti-PA antibodies. Overall, 3 intramuscular immunizations of Sprague Dawley rats with AV7909 were well tolerated, did not induce mortality or any systemic adverse effects, and did not result in any delayed toxicity.
Keywords: AV7909; CPG 7909; animal; anthrax; rats; toxicity; vaccine.
Conflict of interest statement
DECLARATION OF CONFLICT(S) OF INTERESTS
No conflict(s) of interests were declared by the author(s) with respect to the research, authorship, and/or publication of this manuscript.
Figures
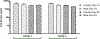
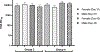
Similar articles
-
Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults.Vaccine. 2016 Apr 19;34(18):2096-105. doi: 10.1016/j.vaccine.2016.03.006. Epub 2016 Mar 12. Vaccine. 2016. PMID: 26979136 Free PMC article. Clinical Trial.
-
Repeat-Dose Toxicity Study of a Lyophilized Recombinant Protective Antigen-Based Anthrax Vaccine Adjuvanted With CpG 7909.Int J Toxicol. 2019 May/Jun;38(3):163-172. doi: 10.1177/1091581819848722. Int J Toxicol. 2019. PMID: 31179828
-
Enhanced early innate and T cell-mediated responses in subjects immunized with Anthrax Vaccine Adsorbed Plus CPG 7909 (AV7909).Vaccine. 2014 Nov 28;32(50):6847-54. doi: 10.1016/j.vaccine.2014.01.096. Epub 2014 Feb 13. Vaccine. 2014. PMID: 24530403 Free PMC article. Clinical Trial.
-
Licensure strategy for pre- and post-exposure prophylaxis of biothrax vaccine: the first vaccine licensed using the FDA animal rule.Expert Rev Vaccines. 2016 Dec;15(12):1467-1479. doi: 10.1080/14760584.2016.1254556. Expert Rev Vaccines. 2016. PMID: 27792416 Review.
-
Vaccines against anthrax based on recombinant protective antigen: problems and solutions.Expert Rev Vaccines. 2019 Aug;18(8):813-828. doi: 10.1080/14760584.2019.1643242. Epub 2019 Aug 6. Expert Rev Vaccines. 2019. PMID: 31298973 Review.
Cited by
-
Anthrax Vaccines in the 21st Century.Vaccines (Basel). 2024 Feb 3;12(2):159. doi: 10.3390/vaccines12020159. Vaccines (Basel). 2024. PMID: 38400142 Free PMC article. Review.
-
Development of Effective Medical Countermeasures Against the Main Biowarfare Agents: The Importance of Antibodies.Microorganisms. 2024 Dec 18;12(12):2622. doi: 10.3390/microorganisms12122622. Microorganisms. 2024. PMID: 39770824 Free PMC article. Review.
-
One Health adjuvant selection for vaccines against zoonotic infections.Explor Med. 2025;6:1001316. doi: 10.37349/emed.2025.1001316. Epub 2025 May 7. Explor Med. 2025. PMID: 40444176 Free PMC article.
References
-
- Qian F, Rausch KM, Muratova O, Zhou H, Song G, Diouf A, Lambert L, Narum DL, Wu Y, Saul A, Miller LH, Long CA, Mullen GE. Addition of CpG ODN to recombinant Pseudomonas aeruginosa ExoProtein A conjugates of AMA1 and Pfs25 greatly increases the number of responders. Vaccine. 2008;26(20):2521–7. - PMC - PubMed
-
- Hu S, Chen H, Ma J, Chen Q, Deng H, Gong F, Huang H, Shi C. CpG7909 adjuvant enhanced immunogenicity efficacy in mice immunized with ESAT6-Ag85A fusion protein, but does not confer significant protection against Mycobacterium tuberculosis infection. J Appl Microbiol 2013;115(5):1203–11. - PubMed
-
- Gu M, Hine PM, James Jackson W, Giri L, Nabors GS. Increased potency of BioThrax anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG 10109. Vaccine. 2007;25(3):526–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

