Aptamer-based biosensors for the diagnosis of sepsis
- PMID: 34281552
- PMCID: PMC8287673
- DOI: 10.1186/s12951-021-00959-5
Aptamer-based biosensors for the diagnosis of sepsis
Abstract
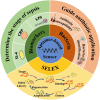
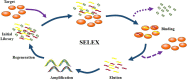
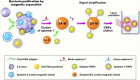
Sepsis, the syndrome of infection complicated by acute organ dysfunction, is a serious and growing global problem, which not only leads to enormous economic losses but also becomes one of the leading causes of mortality in the intensive care unit. The detection of sepsis-related pathogens and biomarkers in the early stage plays a critical role in selecting appropriate antibiotics or other drugs, thereby preventing the emergence of dangerous phases and saving human lives. There are numerous demerits in conventional detection strategies, such as high cost, low efficiency, as well as lacking of sensitivity and selectivity. Recently, the aptamer-based biosensor is an emerging strategy for reasonable sepsis diagnosis because of its accessibility, rapidity, and stability. In this review, we first introduce the screening of suitable aptamer. Further, recent advances of aptamer-based biosensors in the detection of bacteria and biomarkers for the diagnosis of sepsis are summarized. Finally, the review proposes a brief forecast of challenges and future directions with highly promising aptamer-based biosensors.
Keywords: Aptamer-based biosensors; Diagnosis; Nanomaterials; Sepsis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review.Biosens Bioelectron. 2019 Jan 15;124-125:205-215. doi: 10.1016/j.bios.2018.10.034. Epub 2018 Oct 19. Biosens Bioelectron. 2019. PMID: 30388563 Review.
-
Recent Advances in Biological Applications of Aptamer-Based Fluorescent Biosensors.Molecules. 2023 Oct 29;28(21):7327. doi: 10.3390/molecules28217327. Molecules. 2023. PMID: 37959747 Free PMC article. Review.
-
Split aptamer acquisition mechanisms and current application in antibiotics detection: a short review.Crit Rev Food Sci Nutr. 2023;63(28):9098-9110. doi: 10.1080/10408398.2022.2064810. Epub 2022 May 4. Crit Rev Food Sci Nutr. 2023. PMID: 35507474 Review.
-
Recent advances on aptamer-based biosensors to detection of platelet-derived growth factor.Biosens Bioelectron. 2018 Aug 15;113:58-71. doi: 10.1016/j.bios.2018.04.048. Epub 2018 Apr 22. Biosens Bioelectron. 2018. PMID: 29729560 Review.
-
Recent advances on aptamer-based biosensors for detection of pathogenic bacteria.World J Microbiol Biotechnol. 2021 Feb 8;37(3):45. doi: 10.1007/s11274-021-03002-9. World J Microbiol Biotechnol. 2021. PMID: 33554321 Review.
Cited by
-
28-day sepsis mortality prediction model from combined serial interleukin-6, lactate, and procalcitonin measurements: a retrospective cohort study.Eur J Clin Microbiol Infect Dis. 2023 Jan;42(1):77-85. doi: 10.1007/s10096-022-04517-1. Epub 2022 Nov 16. Eur J Clin Microbiol Infect Dis. 2023. PMID: 36383295 Free PMC article.
-
Wave-shaped microfluidic chip assisted point-of-care testing for accurate and rapid diagnosis of infections.Mil Med Res. 2022 Feb 11;9(1):8. doi: 10.1186/s40779-022-00368-1. Mil Med Res. 2022. PMID: 35144683 Free PMC article.
-
The diagnostic utility of IL-10, IL-17, and PCT in patients with sepsis infection.Front Public Health. 2022 Jul 22;10:923457. doi: 10.3389/fpubh.2022.923457. eCollection 2022. Front Public Health. 2022. PMID: 35937269 Free PMC article.
-
Fucoidan-derived carbon dots against Enterococcus faecalis biofilm and infected dentinal tubules for the treatment of persistent endodontic infections.J Nanobiotechnology. 2022 Jul 14;20(1):321. doi: 10.1186/s12951-022-01501-x. J Nanobiotechnology. 2022. PMID: 35836267 Free PMC article.
-
NanoZnO-modified titanium implants for enhanced anti-bacterial activity, osteogenesis and corrosion resistance.J Nanobiotechnology. 2021 Oct 30;19(1):353. doi: 10.1186/s12951-021-01099-6. J Nanobiotechnology. 2021. PMID: 34717648 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- Grant No. 31900957/National Natural Science Foundation of China
- Grant No. ZR2019QC007/Natural Science Foundation of Shandong Province
- Grant No. 2019KJE015/Innovation and technology program for the excellent youth scholars of higher education of Shandong province
- Grant No. 2019M652326/Postdoctoral Research Foundation of China
- S202011065031/National College Students Innovation and Entrepreneurship Training Program
LinkOut - more resources
Full Text Sources
Medical

