Growth rate-dependent flexural rigidity of microtubules influences pattern formation in collective motion
- PMID: 34281555
- PMCID: PMC8287809
- DOI: 10.1186/s12951-021-00960-y
Growth rate-dependent flexural rigidity of microtubules influences pattern formation in collective motion
Abstract
Background: Microtubules (MTs) are highly dynamic tubular cytoskeleton filaments that are essential for cellular morphology and intracellular transport. In vivo, the flexural rigidity of MTs can be dynamically regulated depending on their intracellular function. In the in vitro reconstructed MT-motor system, flexural rigidity affects MT gliding behaviors and trajectories. Despite the importance of flexural rigidity for both biological functions and in vitro applications, there is no clear interpretation of the regulation of MT flexural rigidity, and the results of many studies are contradictory. These discrepancies impede our understanding of the regulation of MT flexural rigidity, thereby challenging its precise manipulation.
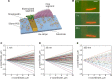
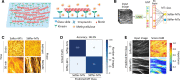
Results: Here, plausible explanations for these discrepancies are provided and a new method to evaluate the MT rigidity is developed. Moreover, a new relationship of the dynamic and mechanic of MTs is revealed that MT flexural rigidity decreases through three phases with the growth rate increases, which offers a method of designing MT flexural rigidity by regulating its growth rate. To test the validity of this method, the gliding performances of MTs with different flexural rigidities polymerized at different growth rates are examined. The growth rate-dependent flexural rigidity of MTs is experimentally found to influence the pattern formation in collective motion using gliding motility assay, which is further validated using machine learning.
Conclusion: Our study establishes a robust quantitative method for measurement and design of MT flexural rigidity to study its influences on MT gliding assays, collective motion, and other biological activities in vitro. The new relationship about the growth rate and rigidity of MTs updates current concepts on the dynamics and mechanics of MTs and provides comparable data for investigating the regulation mechanism of MT rigidity in vivo in the future.
Keywords: Collective motion; Flexural rigidity; Growth rate; Localization precision; Microtubule.
© 2021. The Author(s).
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Mücke N, Kreplak L, Kirmse R, Wedig T, Herrmann H, Aebi U. Assessing the flexibility of intermediate filaments by atomic force microscopy. J Mol Biol. 2004;335:1241–1250. - PubMed
-
- Hawkins T, Mirigian M, Selcuk Yasar M, Ross JL. Mechanics of microtubules. J Biomech. 2010;43:23–30. - PubMed
-
- Sumino Y, Nagai KH, Shitaka Y, Tanaka D, Yoshikawa K, Chaté H. Large-scale vortex lattice emerging from collectively moving microtubules. Nature. 2012;483:448–452. - PubMed

