Normative data on spontaneous stride velocity, stride length, and walking activity in a non-controlled environment
- PMID: 34281599
- PMCID: PMC8287788
- DOI: 10.1186/s13023-021-01956-5
Normative data on spontaneous stride velocity, stride length, and walking activity in a non-controlled environment
Abstract
Background: Normative data are necessary for validation of new outcome measures. Recently, the 95th centile of stride speed was qualified by the European Medicines Agency as a valid secondary outcome for clinical trials in subjects with Duchenne muscular dystrophy. This study aims to obtain normative data on spontaneous stride velocity and length in a non-controlled environment and their evolution after 12 months.
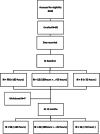
Method: Ninety-one healthy volunteers (50 females, 41 males), with a mean age of 16 years and 2 months, were recruited and assessed at baseline and 12 months later. The 4-stair climb, 6-min walk test, 10-m walk test and rise from floor assessments were performed. Stride length, stride velocity, and the distance walked per hour were studied in an everyday setting for one month after each evaluation.
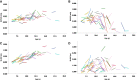
Results: Of the 91 subjects assessed, 82 provided more than 50 h of recordings at baseline; and 73 subjects provided the same at the end of the year. We observed significant positive correlations of the stride length with age and height of participants, and a significant increase of the median stride length in children after the period. In this group, the 95th centile stride velocity was not correlated with age and was stable after one year. All measures but the 10MWT were stable in adults after a one-year period.
Conclusion: This study provides with data on the influence of age, height, and gender on stride velocity and length as well as accounting for natural changes after one year in controls.
Keywords: Accelerometer; Healthy volunteers; Home monitoring; Stride length; Stride velocity.
© 2021. The Author(s).
Conflict of interest statement
Academic authors declare no competing interests. Melanie Annoussamy and Damien Eggenspieler are employed by Synsav, the medium-sized enterprise responsible for ActiMyo® development. David Vissière is the CEO, founder and main shareholder of Sysnav.
Figures
References
-
- Clemens PR, Rao VK, Connolly AM, Harper AD, Mah JK, Smith EC, et al. Safety, tolerability, and efficacy of viltolarsen in boys with duchenne muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol. 2020;77(8):982–991. doi: 10.1001/jamaneurol.2020.1264. - DOI - PMC - PubMed
-
- McDonald CM, Campbell C, Torricelli RE, Finkel RS, Flanigan KM, Goemans N, et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10101):1489–1498. doi: 10.1016/s0140-6736(17)31611-2. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

