The ESCRT-III protein VPS4, but not CHMP4B or CHMP2B, is pathologically increased in familial and sporadic ALS neuronal nuclei
- PMID: 34281622
- PMCID: PMC8287756
- DOI: 10.1186/s40478-021-01228-0
The ESCRT-III protein VPS4, but not CHMP4B or CHMP2B, is pathologically increased in familial and sporadic ALS neuronal nuclei
Abstract
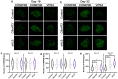
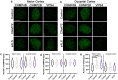
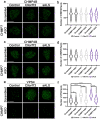
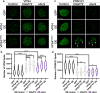
Nuclear pore complex injury has recently emerged as an early and significant contributor to familial and sporadic ALS disease pathogenesis. However, the molecular events leading to this pathological phenomenon characterized by the reduction of specific nucleoporins from neuronal nuclear pore complexes remain largely unknown. This is due in part to a lack of knowledge regarding the biological pathways and proteins underlying nuclear pore complex homeostasis specifically in human neurons. We have recently uncovered that aberrant nuclear accumulation of the ESCRT-III protein CHMP7 initiates nuclear pore complex in familial and sporadic ALS neurons. In yeast and non-neuronal mammalian cells, nuclear relocalization of CHMP7 has been shown to recruit the ESCRT-III proteins CHMP4B, CHMP2B, and VPS4 to facilitate nuclear pore complex and nuclear envelope repair and homeostasis. Here, using super resolution structured illumination microscopy, we find that neither CHMP4B nor CHMP2B are increased in ALS neuronal nuclei. In contrast, VPS4 expression is significantly increased in ALS neuronal nuclei prior to the emergence of nuclear pore injury in a CHMP7 dependent manner. However, unlike our prior CHMP7 knockdown studies, impaired VPS4 function does not mitigate alterations to the NPC and the integral transmembrane nucleoporin POM121. Collectively our data suggest that while alterations in VPS4 subcellular localization appear to be coincident with nuclear pore complex injury, therapeutic efforts to mitigate this pathogenic cascade should be targeted towards upstream events such as the nuclear accumulation of CHMP7 as we have previously described.
Keywords: ALS; CHMP2B; CHMP4B; CHMP7; ESCRT-III; FTD; Nuclear pore complex; Nucleoporins; POM121; VPS4.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
CHMP2B promotes CHMP7 mediated nuclear pore complex injury in sporadic ALS.Acta Neuropathol Commun. 2024 Dec 21;12(1):199. doi: 10.1186/s40478-024-01916-7. Acta Neuropathol Commun. 2024. PMID: 39709457 Free PMC article.
-
SUN1 facilitates CHMP7 nuclear influx and injury cascades in sporadic amyotrophic lateral sclerosis.Brain. 2024 Jan 4;147(1):109-121. doi: 10.1093/brain/awad291. Brain. 2024. PMID: 37639327 Free PMC article.
-
Nuclear accumulation of CHMP7 initiates nuclear pore complex injury and subsequent TDP-43 dysfunction in sporadic and familial ALS.Sci Transl Med. 2021 Jul 28;13(604):eabe1923. doi: 10.1126/scitranslmed.abe1923. Sci Transl Med. 2021. PMID: 34321318 Free PMC article.
-
Structure and mechanism of the ESCRT pathway AAA+ ATPase Vps4.Biochem Soc Trans. 2019 Feb 28;47(1):37-45. doi: 10.1042/BST20180260. Epub 2019 Jan 15. Biochem Soc Trans. 2019. PMID: 30647138 Free PMC article. Review.
-
Emerging Connections between Nuclear Pore Complex Homeostasis and ALS.Int J Mol Sci. 2022 Jan 25;23(3):1329. doi: 10.3390/ijms23031329. Int J Mol Sci. 2022. PMID: 35163252 Free PMC article. Review.
Cited by
-
Nucleoporins are degraded via upregulation of ESCRT-III/Vps4 complex in Drosophila models of C9-ALS/FTD.Cell Rep. 2022 Sep 20;40(12):111379. doi: 10.1016/j.celrep.2022.111379. Cell Rep. 2022. PMID: 36130523 Free PMC article.
-
Emerging Role of Astrocyte-Derived Extracellular Vesicles as Active Participants in CNS Neuroimmune Responses.Immunol Invest. 2024 Jan;53(1):26-39. doi: 10.1080/08820139.2023.2281621. Epub 2023 Nov 19. Immunol Invest. 2024. PMID: 37981468 Free PMC article. Review.
-
CHMP2B promotes CHMP7 mediated nuclear pore complex injury in sporadic ALS.Acta Neuropathol Commun. 2024 Dec 21;12(1):199. doi: 10.1186/s40478-024-01916-7. Acta Neuropathol Commun. 2024. PMID: 39709457 Free PMC article.
-
Nuclear and degradative functions of the ESCRT-III pathway: implications for neurodegenerative disease.Nucleus. 2024 Dec;15(1):2349085. doi: 10.1080/19491034.2024.2349085. Epub 2024 May 3. Nucleus. 2024. PMID: 38700207 Free PMC article. Review.
-
Nuclear pore complexes - a doorway to neural injury in neurodegeneration.Nat Rev Neurol. 2022 Jun;18(6):348-362. doi: 10.1038/s41582-022-00653-6. Epub 2022 Apr 29. Nat Rev Neurol. 2022. PMID: 35488039 Free PMC article. Review.
References
-
- Clayton EL, Mizielinska S, Edgar JR, Nielsen TT, Marshall S, Norona FE, Robbins M, Damirji H, Holm IE, Johannsen P, et al. Frontotemporal dementia caused by CHMP2B mutation is characterised by neuronal lysosomal storage pathology. Acta Neuropathol. 2015;130:511–523. doi: 10.1007/s00401-015-1475-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

