Hypoactive Thalamic Crh+ Cells in a Female Mouse Model of Alcohol Drinking After Social Trauma
- PMID: 34281710
- PMCID: PMC8463500
- DOI: 10.1016/j.biopsych.2021.05.022
Hypoactive Thalamic Crh+ Cells in a Female Mouse Model of Alcohol Drinking After Social Trauma
Abstract
Background: Comorbid stress-induced mood and alcohol use disorders are increasingly prevalent among female patients. Stress exposure can disrupt salience processing and goal-directed decision making, contributing to persistent maladaptive behavioral patterns; these and other stress-sensitive cognitive and behavioral processes rely on dynamic and coordinated signaling by midline and intralaminar thalamic nuclei. Considering the role of social trauma in the trajectory of these debilitating psychopathologies, identifying vulnerable thalamic cells may provide guidance for targeting persistent stress-induced symptoms.
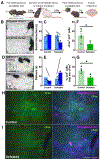
Methods: A novel behavioral protocol traced the progression from social trauma to the development of social defensiveness and chronically escalated alcohol consumption in female mice. Recent cell activation-measured as cFos-was quantified in thalamic cells after safe social interactions, revealing stress-sensitive corticotropin-releasing hormone-expressing (Crh+) anterior central medial thalamic (aCMT) cells. These cells were optogenetically stimulated during stress-induced social defensiveness and abstinence-escalated binge drinking.
Results: Crh+ aCMT neurons exhibited substantial activation after social interactions in stress-naïve but not in stressed female mice. Photoactivating Crh+ aCMT cells dampened stress-induced social deficits, whereas inhibiting these cells increased social defensiveness in stress-naïve mice. Optogenetically activating Crh+ aCMT cells diminished abstinence-escalated binge alcohol drinking in female mice, regardless of stress history.
Conclusions: This work uncovers a role for Crh+ aCMT neurons in maladaptive stress-induced social interactions and in binge drinking after forced abstinence in female mice. This molecularly defined thalamic cell population may serve as a critical stress-sensitive hub for social deficits caused by exposure to social trauma and for patterns of excessive alcohol drinking in female populations.
Keywords: Alcohol; Corticotropin-releasing hormone; Defensive behavior; Female chronic social defeat stress; Social interaction; Thalamus.
Copyright © 2021 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
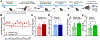


Comment in
-
Social Stress-Induced Behavioral Adaptations in Females: A Focus on Midline Thalamus Crh Neurons.Biol Psychiatry. 2021 Oct 15;90(8):513-515. doi: 10.1016/j.biopsych.2021.08.004. Biol Psychiatry. 2021. PMID: 34556205 No abstract available.
References
-
- Kessler RC (2003): Epidemiology of women and depression. J Affect Disord. 74:5–13. - PubMed
-
- Kessler RC (1997): The effects of stressful life events on depression. Annu Rev Psychol. 48:191–214. - PubMed
-
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995): Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 52:1048–1060. - PubMed
-
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB (1993): Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 29:85–96. - PubMed
-
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR (1997): Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 54:1044–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

