HECT E3 Ubiquitin Ligase Nedd4 Is Required for Antifungal Innate Immunity
- PMID: 34282001
- PMCID: PMC8324540
- DOI: 10.4049/jimmunol.2100083
HECT E3 Ubiquitin Ligase Nedd4 Is Required for Antifungal Innate Immunity
Abstract
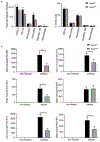
Candida albicans is the most common cause of fungal infections in humans, and disseminated candidiasis has become one of the leading causes of hospital-acquired bloodstream infections with a high mortality rate. However, little is known about the host-pathogen interactions and the mechanisms of antifungal immunity. Here, we report that Nedd4 (neuronal precursor cell-expressed developmentally downregulated 4) is essential for signaling through Dectin-1 and Dectin-2/3. We showed that mice that lack Nedd4 globally or only in the myeloid compartment are highly susceptible to systemic C. albicans infection, which correlates with heightened organ fungal burden, defective inflammatory response, impaired leukocyte recruitment to the kidneys, and defective reactive oxygen species expression by granulocytes. At the molecular level, Nedd4 -/- macrophages displayed impaired activation of TGF-β-activating kinase-1 and NF-κB, but normal activation of spleen tyrosine kinase and protein kinase C-δ on C. albicans yeast and hyphal infections. These data suggest that Nedd4 regulates signaling events downstream of protein kinase C-δ but upstream of or at TGF-β-activating kinase-1.
Copyright © 2021 by The American Association of Immunologists, Inc.
Figures




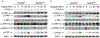
Similar articles
-
Phagocytes from Mice Lacking the Sts Phosphatases Have an Enhanced Antifungal Response to Candida albicans.mBio. 2018 Jul 17;9(4):e00782-18. doi: 10.1128/mBio.00782-18. mBio. 2018. PMID: 30018105 Free PMC article.
-
Targeting CBLB as a potential therapeutic approach for disseminated candidiasis.Nat Med. 2016 Aug;22(8):906-14. doi: 10.1038/nm.4141. Epub 2016 Jul 18. Nat Med. 2016. PMID: 27428899 Free PMC article.
-
MYO1F regulates antifungal immunity by regulating acetylation of microtubules.Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2100230118. doi: 10.1073/pnas.2100230118. Proc Natl Acad Sci U S A. 2021. PMID: 34301894 Free PMC article.
-
The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives.Mol Med. 2023 Mar 14;29(1):34. doi: 10.1186/s10020-023-00628-3. Mol Med. 2023. PMID: 36918822 Free PMC article. Review.
-
Modulating Host Signaling Pathways to Promote Resistance to Infection by Candida albicans.Front Cell Infect Microbiol. 2017 Nov 21;7:481. doi: 10.3389/fcimb.2017.00481. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29201860 Free PMC article. Review.
Cited by
-
K27-linked RORγt ubiquitination by Nedd4 potentiates Th17-mediated autoimmunity.J Biomed Sci. 2025 Feb 19;32(1):26. doi: 10.1186/s12929-025-01120-2. J Biomed Sci. 2025. PMID: 39972304 Free PMC article.
-
Reprogramming of tumor-associated macrophages via NEDD4-mediated CSF1R degradation by targeting USP18.Cell Rep. 2023 Dec 26;42(12):113560. doi: 10.1016/j.celrep.2023.113560. Epub 2023 Dec 13. Cell Rep. 2023. PMID: 38100351 Free PMC article.
-
Akt-2 Is a Potential Therapeutic Target for Disseminated Candidiasis.J Immunol. 2022 Sep 1;209(5):991-1000. doi: 10.4049/jimmunol.2101003. Epub 2022 Aug 5. J Immunol. 2022. PMID: 36130126 Free PMC article.
References
-
- Havlickova B, Czaika VA, and Friedrich M. 2008. Epidemiological trends in skin mycoses worldwide. Mycoses 51 Suppl 4: 2–15. - PubMed
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, and White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4: 165rv113. - PubMed
-
- Lionakis MS, and Levitz SM. 2018. Host Control of Fungal Infections: Lessons from Basic Studies and Human Cohorts. Annu Rev Immunol 36: 157–191. - PubMed
-
- Antinori S, Milazzo L, Sollima S, Galli M, and Corbellino M. 2016. Candidemia and invasive candidiasis in adults: A narrative review. Eur J Intern Med 34: 21–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

