Genetic Screens Identify a Context-Specific PI3K/p27Kip1 Node Driving Extrahepatic Biliary Cancer
- PMID: 34282029
- PMCID: PMC7612573
- DOI: 10.1158/2159-8290.CD-21-0209
Genetic Screens Identify a Context-Specific PI3K/p27Kip1 Node Driving Extrahepatic Biliary Cancer
Abstract
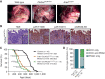
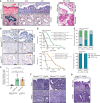
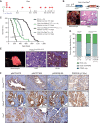
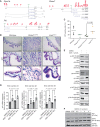
Biliary tract cancer ranks among the most lethal human malignancies, representing an unmet clinical need. Its abysmal prognosis is tied to an increasing incidence and a fundamental lack of mechanistic knowledge regarding the molecular basis of the disease. Here, we show that the Pdx1-positive extrahepatic biliary epithelium is highly susceptible toward transformation by activated PIK3CAH1047R but refractory to oncogenic KrasG12D. Using genome-wide transposon screens and genetic loss-of-function experiments, we discover context-dependent genetic interactions that drive extrahepatic cholangiocarcinoma (ECC) and show that PI3K signaling output strength and repression of the tumor suppressor p27Kip1 are critical context-specific determinants of tumor formation. This contrasts with the pancreas, where oncogenic Kras in concert with p53 loss is a key cancer driver. Notably, inactivation of p27Kip1 permits KrasG12D-driven ECC development. These studies provide a mechanistic link between PI3K signaling, tissue-specific tumor suppressor barriers, and ECC pathogenesis, and present a novel genetic model of autochthonous ECC and genes driving this highly lethal tumor subtype.
Significance: We used the first genetically engineered mouse model for extrahepatic bile duct carcinoma to identify cancer genes by genome-wide transposon-based mutagenesis screening. Thereby, we show that PI3K signaling output strength and p27Kip1 function are critical determinants for context-specific ECC formation. This article is highlighted in the In This Issue feature, p. 2945.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
- doi: 10.1158/2159-8290.CD-11-12-ITI
Similar articles
-
p53 protects against formation of extrahepatic biliary precancerous lesions in the context of oncogenic Kras.Oncotarget. 2023 Mar 31;14:276-279. doi: 10.18632/oncotarget.28380. Oncotarget. 2023. PMID: 36999984 Free PMC article.
-
Oncogenic KRAS-expressing organoids with biliary epithelial stem cell properties give rise to biliary tract cancer in mice.Cancer Sci. 2021 May;112(5):1822-1838. doi: 10.1111/cas.14703. Epub 2021 Mar 8. Cancer Sci. 2021. PMID: 33068050 Free PMC article.
-
Simultaneous activation of Kras-Akt and Notch pathways induces extrahepatic biliary cancer via the mTORC1 pathway.J Pathol. 2023 Aug;260(4):478-492. doi: 10.1002/path.6139. Epub 2023 Jun 13. J Pathol. 2023. PMID: 37310065
-
Biliary Tract Cancer: Molecular Biology of Precursor Lesions.Semin Liver Dis. 2023 Nov;43(4):472-484. doi: 10.1055/a-2207-9834. Epub 2023 Nov 9. Semin Liver Dis. 2023. PMID: 37944999 Review.
-
[Gallbladder and bile duct carcinoma. Biology and pathology].Internist (Berl). 2004 Jan;45(1):33-41. doi: 10.1007/s00108-003-1110-6. Internist (Berl). 2004. PMID: 14735242 Review. German.
Cited by
-
Selecting an Appropriate Experimental Animal Model for Cholangiocarcinoma Research.J Clin Transl Hepatol. 2022 Aug 28;10(4):700-710. doi: 10.14218/JCTH.2021.00374. Epub 2022 Feb 11. J Clin Transl Hepatol. 2022. PMID: 36062286 Free PMC article. Review.
-
Criteria for preclinical models of cholangiocarcinoma: scientific and medical relevance.Nat Rev Gastroenterol Hepatol. 2023 Jul;20(7):462-480. doi: 10.1038/s41575-022-00739-y. Epub 2023 Feb 8. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36755084 Review.
-
Genomic profiling of idiopathic peri-hilar cholangiocarcinoma reveals new targets and mutational pathways.Sci Rep. 2023 Apr 24;13(1):6681. doi: 10.1038/s41598-023-33096-0. Sci Rep. 2023. PMID: 37095160 Free PMC article.
-
Cholangiocarcinoma: Molecular Abnormalities and Cells of Origin.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338221128689. doi: 10.1177/15330338221128689. Technol Cancer Res Treat. 2023. PMID: 36872875 Free PMC article. Review.
-
PTEN deficiency induces an extrahepatic cholangitis-cholangiocarcinoma continuum via aurora kinase A in mice.J Hepatol. 2024 Jul;81(1):120-134. doi: 10.1016/j.jhep.2024.02.018. Epub 2024 Feb 28. J Hepatol. 2024. PMID: 38428643 Free PMC article.
References
-
- Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato Met al. . Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

