Incidental findings from cancer next generation sequencing panels
- PMID: 34282142
- PMCID: PMC8289933
- DOI: 10.1038/s41525-021-00224-6
Incidental findings from cancer next generation sequencing panels
Abstract
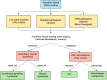
Next-generation sequencing (NGS) technologies have facilitated multi-gene panel (MGP) testing to detect germline DNA variants in hereditary cancer patients. This sensitive technique can uncover unexpected, non-germline incidental findings indicative of mosaicism, clonal hematopoiesis (CH), or hematologic malignancies. A retrospective chart review was conducted to identify cases of incidental findings from NGS-MGP testing. Inclusion criteria included: 1) multiple pathogenic variants in the same patient; 2) pathogenic variants at a low allele fraction; and/or 3) the presence of pathogenic variants not consistent with family history. Secondary tissue analysis, complete blood count (CBC) and medical record review were conducted to further delineate the etiology of the pathogenic variants. Of 6060 NGS-MGP tests, 24 cases fulfilling our inclusion criteria were identified. Pathogenic variants were detected in TP53, ATM, CHEK2, BRCA1 and APC. 18/24 (75.0%) patients were classified as CH, 3/24 (12.5%) as mosaic, 2/24 (8.3%) related to a hematologic malignancy, and 1/24 (4.2%) as true germline. We describe a case-specific workflow to identify and interpret the nature of incidental findings on NGS-MGP. This workflow will provide oncology and genetic clinics a practical guide for the management and counselling of patients with unexpected NGS-MGP findings.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

