Methylene blue can act as an antidote to pesticide poisoning of bumble bee mitochondria
- PMID: 34282204
- PMCID: PMC8289979
- DOI: 10.1038/s41598-021-94231-3
Methylene blue can act as an antidote to pesticide poisoning of bumble bee mitochondria
Abstract
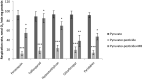
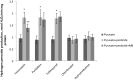
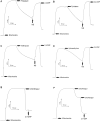
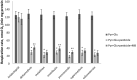
The population of bumble bees and other pollinators has considerably declined worldwide, probably, due to the toxic effect of pesticides used in agriculture. Inexpensive and available antidotes can be one of the solutions for the problem of pesticide toxicity for pollinators. We studied the properties of the thiazine dye Methylene blue (MB) as an antidote against the toxic action of pesticides in the bumble bee mitochondria and found that MB stimulated mitochondrial respiration mediated by Complex I of the electron transport chain (ETC) and increased respiration of the mitochondria treated with mitochondria-targeted (chlorfenapyr, hydramethylnon, pyridaben, tolfenpyrad, and fenazaquin) and non-mitochondrial (deltamethrin, metribuzin, and penconazole) pesticides. MB also restored the mitochondrial membrane potential dissipated by the pesticides affecting the ETC. The mechanism of MB action is most probably related to its ability to shunt electron flow in the mitochondrial ETC.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Enhanced hydrogen peroxide generation accompanies the beneficial bioenergetic effects of methylene blue in isolated brain mitochondria.Free Radic Biol Med. 2014 Dec;77:317-30. doi: 10.1016/j.freeradbiomed.2014.09.024. Epub 2014 Sep 30. Free Radic Biol Med. 2014. PMID: 25277417
-
Methylene blue stimulates substrate-level phosphorylation catalysed by succinyl-CoA ligase in the citric acid cycle.Neuropharmacology. 2017 Sep 1;123:287-298. doi: 10.1016/j.neuropharm.2017.05.009. Epub 2017 May 8. Neuropharmacology. 2017. PMID: 28495375
-
Toxic responses of blue orchard mason bees (Osmia lignaria) following contact exposure to neonicotinoids, macrocyclic lactones, and pyrethroids.Ecotoxicol Environ Saf. 2021 Jan 15;208:111681. doi: 10.1016/j.ecoenv.2020.111681. Epub 2020 Nov 21. Ecotoxicol Environ Saf. 2021. PMID: 33396013
-
Decline and conservation of bumble bees.Annu Rev Entomol. 2008;53:191-208. doi: 10.1146/annurev.ento.53.103106.093454. Annu Rev Entomol. 2008. PMID: 17803456 Review.
-
Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: Methylene blue connects the dots.Prog Neurobiol. 2017 Oct;157:273-291. doi: 10.1016/j.pneurobio.2015.10.005. Epub 2015 Nov 18. Prog Neurobiol. 2017. PMID: 26603930 Free PMC article. Review.
Cited by
-
In vitro larval rearing method of eusocial bumblebee Bombus terrestris for toxicity test.Sci Rep. 2022 Sep 22;12(1):15783. doi: 10.1038/s41598-022-19965-0. Sci Rep. 2022. PMID: 36138070 Free PMC article.
-
Succinate prodrugs in combination with atropine and pralidoxime protect cerebral mitochondrial function in a rodent model of acute organophosphate poisoning.Sci Rep. 2022 Nov 25;12(1):20329. doi: 10.1038/s41598-022-24472-3. Sci Rep. 2022. PMID: 36434021 Free PMC article.
-
Methylene blue at recommended concentrations alters metabolism in early zebrafish development.Commun Biol. 2025 Jan 25;8(1):120. doi: 10.1038/s42003-025-07471-8. Commun Biol. 2025. PMID: 39856203 Free PMC article.
-
Advances in Photodynamic Treatment of Precancerous and Cancerous Gynecological Diseases.Cancers (Basel). 2025 Jul 22;17(15):2421. doi: 10.3390/cancers17152421. Cancers (Basel). 2025. PMID: 40805127 Free PMC article. Review.
-
Degradation strategies of pesticide residue: From chemicals to synthetic biology.Synth Syst Biotechnol. 2023 Mar 23;8(2):302-313. doi: 10.1016/j.synbio.2023.03.005. eCollection 2023 Jun. Synth Syst Biotechnol. 2023. PMID: 37122957 Free PMC article.
References
-
- Connelly H, Poveda K, Loeb G. Landscape simplification decreases wild bee pollination services to strawberry. Agr. Ecosyst. Environ. 2015;211:51–56. doi: 10.1016/j.agee.2015.05.004. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

