Blood glutamate scavengers increase pro-apoptotic signaling and reduce metastatic melanoma growth in-vivo
- PMID: 34282238
- PMCID: PMC8290021
- DOI: 10.1038/s41598-021-94183-8
Blood glutamate scavengers increase pro-apoptotic signaling and reduce metastatic melanoma growth in-vivo
Abstract
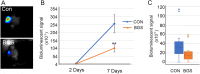
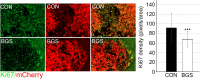
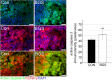
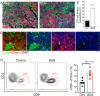
Inhibition of extracellular glutamate (Glu) release decreases proliferation and invasion, induces apoptosis, and inhibits melanoma metastatic abilities. Previous studies have shown that Blood-glutamate scavenging (BGS), a novel treatment approach, has been found to be beneficial in attenuating glioblastoma progression by reducing brain Glu levels. Therefore, in this study we evaluated the ability of BGS treatment to inhibit brain metastatic melanoma progression in-vivo. RET melanoma cells were implanted in C56BL/6J mice to induce brain melanoma tumors followed by treatment with BGS or vehicle administered for fourteen days. Bioluminescent imaging was conducted to evaluate tumor growth, and plasma/CSF Glu levels were monitored throughout. Immunofluorescence staining of Ki67 and 53BP1 was used to analyze tumor cell proliferation and DNA double-strand breaks. In addition, we analyzed CD8, CD68, CD206, p-STAT1 and iNOS expression to evaluate alterations in tumor micro-environment and anti-tumor immune response due to treatment. Our results show that BGS treatment reduces CSF Glu concentration and consequently melanoma growth in-vivo by decreasing tumor cell proliferation and increasing pro-apoptotic signaling in C56BL/6J mice. Furthermore, BGS treatment supported CD8+ cell recruitment and CD68+ macrophage invasion. These findings suggest that BGS can be of potential therapeutic relevance in the treatment of metastatic melanoma.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Blood Glutamate Scavenger as a Novel Neuroprotective Treatment in Spinal Cord Injury.J Neurotrauma. 2018 Nov 1;35(21):2581-2590. doi: 10.1089/neu.2017.5524. Epub 2018 May 15. J Neurotrauma. 2018. PMID: 29495919
-
Blood glutamate scavengers prolong the survival of rats and mice with brain-implanted gliomas.Invest New Drugs. 2012 Dec;30(6):2226-35. doi: 10.1007/s10637-012-9799-5. Invest New Drugs. 2012. PMID: 22392507 Free PMC article.
-
Concurrent Targeting of Glutaminolysis and Metabotropic Glutamate Receptor 1 (GRM1) Reduces Glutamate Bioavailability in GRM1+ Melanoma.Cancer Res. 2019 Apr 15;79(8):1799-1809. doi: 10.1158/0008-5472.CAN-18-1500. Epub 2019 Apr 15. Cancer Res. 2019. PMID: 30987979 Free PMC article.
-
The current management of brain metastasis in melanoma: a focus on riluzole.Expert Rev Neurother. 2015;15(7):779-92. doi: 10.1586/14737175.2015.1055321. Epub 2015 Jun 19. Expert Rev Neurother. 2015. PMID: 26092602 Review.
-
Immune Responses to BRAF-Targeted Therapy in Melanoma: Is Targeted Therapy Immunotherapy?Crit Rev Oncog. 2016;21(1-2):83-91. doi: 10.1615/CritRevOncog.2016017150. Crit Rev Oncog. 2016. PMID: 27481005 Review.
Cited by
-
Atherosis-associated lnc_000048 activates PKR to enhance STAT1-mediated polarization of THP-1 macrophages to M1 phenotype.Neural Regen Res. 2024 Nov 1;19(11):2488-2498. doi: 10.4103/NRR.NRR-D-23-01355. Epub 2024 Mar 8. Neural Regen Res. 2024. PMID: 38526285 Free PMC article.
-
Electroporation-based proteome sampling ex vivo enables the detection of brain melanoma protein signatures in a location proximate to visible tumor margins.PLoS One. 2022 May 19;17(5):e0265866. doi: 10.1371/journal.pone.0265866. eCollection 2022. PLoS One. 2022. PMID: 35588133 Free PMC article.
-
Neuronal Cannabinoid CB1 Receptors Suppress the Growth of Melanoma Brain Metastases by Inhibiting Glutamatergic Signalling.Cancers (Basel). 2023 Apr 24;15(9):2439. doi: 10.3390/cancers15092439. Cancers (Basel). 2023. PMID: 37173906 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

