Solid stress impairs lymphocyte infiltration into lymph-node metastases
- PMID: 34282290
- PMCID: PMC8678215
- DOI: 10.1038/s41551-021-00766-1
Solid stress impairs lymphocyte infiltration into lymph-node metastases
Abstract
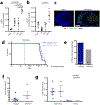
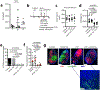
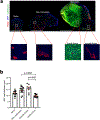
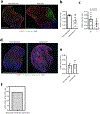
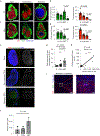
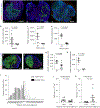
Strong and durable anticancer immune responses are associated with the generation of activated cancer-specific T cells in the draining lymph nodes. However, cancer cells can colonize lymph nodes and drive tumour progression. Here, we show that lymphocytes fail to penetrate metastatic lesions in lymph nodes. In tissue from patients with breast, colon, and head and neck cancers, as well as in mice with spontaneously developing breast-cancer lymph-node metastases, we found that lymphocyte exclusion from nodal lesions is associated with the presence of solid stress caused by lesion growth, that solid stress induces reductions in the number of functional high endothelial venules in the nodes, and that relieving solid stress in the mice increased the presence of lymphocytes in lymph-node lesions by about 15-fold. Solid-stress-mediated impairment of lymphocyte infiltration into lymph-node metastases suggests a therapeutic route for overcoming T-cell exclusion during immunotherapy.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




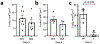






References
-
- Jatoi I, Hilsenbeck SG, Clark GM & Osborne CK Significance of axillary lymph node metastasis in primary breast cancer. J. Clin. Oncol 17, 2334–2340 (1999). - PubMed
-
- Nathanson SD, Kwon D, Kapke A, Alford SH & Chitale D The role of lymph node metastasis in the systemic dissemination of breast cancer. Ann. Surg. Oncol 16, 3396–3405 (2009). - PubMed
-
- Brown M et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359, 1408–1411 (2018). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

