BATF regulates progenitor to cytolytic effector CD8+ T cell transition during chronic viral infection
- PMID: 34282329
- PMCID: PMC9258987
- DOI: 10.1038/s41590-021-00965-7
BATF regulates progenitor to cytolytic effector CD8+ T cell transition during chronic viral infection
Abstract
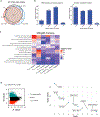
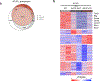
During chronic viral infection, CD8+ T cells develop into three major phenotypically and functionally distinct subsets: Ly108+TCF-1+ progenitors, Ly108-CX3CR1- terminally exhausted cells and the recently identified CX3CR1+ cytotoxic effector cells. Nevertheless, how CX3CR1+ effector cell differentiation is transcriptionally and epigenetically regulated remains elusive. Here, we identify distinct gene regulatory networks and epigenetic landscapes underpinning the formation of these subsets. Notably, our data demonstrate that CX3CR1+ effector cells bear a striking similarity to short-lived effector cells during acute infection. Genetic deletion of Tbx21 significantly diminished formation of the CX3CR1+ subset. Importantly, we further identify a previously unappreciated role for the transcription factor BATF in maintaining a permissive chromatin structure that allows the transition from TCF-1+ progenitors to CX3CR1+ effector cells. BATF directly bound to regulatory regions near Tbx21 and Klf2, modulating their enhancer accessibility to facilitate the transition. These mechanistic insights can potentially be harnessed to overcome T cell exhaustion during chronic infection and cancer.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures













Comment in
-
BATF targets T cell exhaustion for termination.Nat Immunol. 2021 Aug;22(8):936-938. doi: 10.1038/s41590-021-00978-2. Nat Immunol. 2021. PMID: 34282328 Free PMC article.
References
-
- He R et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 537, 412–428 (2016). - PubMed
-
- Utzschneider DT et al. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016). - PubMed
-
- Leong YA et al. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol. 17, 1187–1196 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

