COVIDTrach: a prospective cohort study of mechanically ventilated patients with COVID-19 undergoing tracheostomy in the UK
- PMID: 34282409
- PMCID: PMC8275367
- DOI: 10.1136/bmjsit-2020-000077
COVIDTrach: a prospective cohort study of mechanically ventilated patients with COVID-19 undergoing tracheostomy in the UK
Abstract
Objectives: COVIDTrach is a UK multicentre prospective cohort study project that aims to evaluate the outcomes of tracheostomy in patients with COVID-19 receiving mechanical ventilation and record the incidence of SARS-CoV-2 infection among healthcare workers involved in the procedure.
Design: Data on patient demographic, clinical history and outcomes were entered prospectively and updated over time via an online database (REDCap). Clinical variables were compared with outcomes, with logistic regression used to develop a model for mortality. Participants recorded whether any operators tested positive for SARS-CoV-2 within 2 weeks of the procedure.
Setting: UK National Health Service departments involved in treating patients with COVID-19 receiving mechanical ventilation.
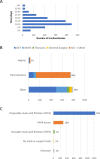
Participants: The cohort comprised 1605 tracheostomy cases from 126 UK hospitals collected between 6 April and 26 August 2020.
Main outcome measures: Mortality following tracheostomy, successful wean from mechanical ventilation and length of time from tracheostomy to wean, discharge from hospital, complications from tracheostomy, reported SARS-CoV-2 infection among operators.
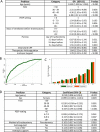
Results: The median time from intubation to tracheostomy was 15 days (IQR 11, 21). 285 (18%) patients died following the procedure. 1229 (93%) of the survivors had been successfully weaned from mechanical ventilation at censoring and 1049 (81%) had been discharged from hospital. Age, inspired oxygen concentration, positive end-expiratory pressure setting, fever, number of days of ventilation before tracheostomy, C reactive protein and the use of anticoagulation and inotropic support independently predicted mortality. Six reports were received of operators testing positive for SARS-CoV-2 within 2 weeks of the procedure.
Conclusions: Tracheostomy appears to be safe in mechanically ventilated patients with COVID-19 and to operators performing the procedure and we identified clinical parameters that are predictive of mortality.
Trial registration number: The study is registered with ClinicalTrials.Gov (NCT04572438).
Keywords: Anaesthesiology Devices; COVID-19; cohort study; ear–nose–throat devices.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
