This is a preprint.
Myeloid cell interferon responses correlate with clearance of SARS-CoV-2
- PMID: 34282414
- PMCID: PMC8288154
- DOI: 10.21203/rs.3.rs-664507/v1
Myeloid cell interferon responses correlate with clearance of SARS-CoV-2
Update in
-
Myeloid cell interferon responses correlate with clearance of SARS-CoV-2.Nat Commun. 2022 Feb 3;13(1):679. doi: 10.1038/s41467-022-28315-7. Nat Commun. 2022. PMID: 35115549 Free PMC article.
Abstract
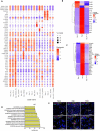
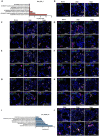
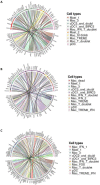
The emergence of mutant SARS-CoV-2 strains associated with an increased risk of COVID-19-related death necessitates better understanding of the early viral dynamics, host responses and immunopathology. While studies have reported immune profiling using single cell RNA sequencing in terminal human COVID-19 patients, performing longitudinal immune cell dynamics in humans is challenging. Macaques are a suitable model of SARS-CoV-2 infection. We performed longitudinal single-cell RNA sequencing of bronchoalveolar lavage (BAL) cell suspensions from adult rhesus macaques infected with SARS-CoV-2 (n=6) to delineate the early dynamics of immune cells changes. The bronchoalveolar compartment exhibited dynamic changes in transcriptional landscape 3 days post- SARS-CoV-2-infection (3dpi) (peak viremia), relative to 14-17dpi (recovery phase) and pre-infection (baseline). We observed the accumulation of distinct populations of both macrophages and T-lymphocytes expressing strong interferon-driven inflammatory gene signature at 3dpi. Type I IFN response was highly induced in the plasmacytoid dendritic cells. The presence of a distinct HLADR+CD68+CD163+SIGLEC1+ macrophage population exhibiting higher angiotensin converting enzyme 2 (ACE2) expression was also observed. These macrophages were significantly recruited to the lungs of macaques at 3dpi and harbored SARS-CoV-2, while expressing a strong interferon-driven innate anti-viral gene signature. The accumulation of these responses correlated with decline in viremia and recovery. The recruitment of a myeloid cell-mediated Type I IFN response is associated with the rapid clearance of SARS-CoV-2 infection in macaques.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

