Repurposing eflornithine to treat a patient with a rare ODC1 gain-of-function variant disease
- PMID: 34282722
- PMCID: PMC8291972
- DOI: 10.7554/eLife.67097
Repurposing eflornithine to treat a patient with a rare ODC1 gain-of-function variant disease
Abstract
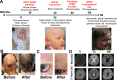
Background: Polyamine levels are intricately controlled by biosynthetic, catabolic enzymes and antizymes. The complexity suggests that minute alterations in levels lead to profound abnormalities. We described the therapeutic course for a rare syndrome diagnosed by whole exome sequencing caused by gain-of-function variants in the C-terminus of ornithine decarboxylase (ODC), characterized by neurological deficits and alopecia.
Methods: N-acetylputrescine levels with other metabolites were measured using ultra-performance liquid chromatography paired with mass spectrometry and Z-scores established against a reference cohort of 866 children.
Results: From previous studies and metabolic profiles, eflornithine was identified as potentially beneficial with therapy initiated on FDA approval. Eflornithine normalized polyamine levels without disrupting other pathways. She demonstrated remarkable improvement in both neurological symptoms and cortical architecture. She gained fine motor skills with the capacity to feed herself and sit with support.
Conclusions: This work highlights the strategy of repurposing drugs to treat a rare disease.
Funding: No external funding was received for this work.
Keywords: genetics; genomics; global metabolomics; human; repurposing drugs; whole exome sequencing.
© 2021, Rajasekaran et al.
Conflict of interest statement
SR, CB, AB Inventor on a pending US patent (US-2020215010-A1), methods for treating or preventing developmental disorders associated with mutations in the OCD1 gene. ML, AS, CR, JJ, EG, EV, YE, BW, JP No competing interests declared
Figures

References
-
- Alirol E, Schrumpf D, Amici Heradi J, Riedel A, de Patoul C, Quere M, Chappuis F. Nifurtimox-Eflornithine Combination Therapy for Second-Stage Gambiense Human African Trypanosomiasis: Médecins Sans Frontières Experience in the Democratic Republic of the Congo. Clinical Infectious Diseases. 2013;56:195–203. doi: 10.1093/cid/cis886. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

