Potent Anticancer Effects of Epidithiodiketopiperazine NT1721 in Cutaneous T Cell Lymphoma
- PMID: 34282785
- PMCID: PMC8268131
- DOI: 10.3390/cancers13133367
Potent Anticancer Effects of Epidithiodiketopiperazine NT1721 in Cutaneous T Cell Lymphoma
Erratum in
-
Correction: Lin et al. Potent Anticancer Effects of Epidithiodiketopiperazine NT1721 in Cutaneous T Cell Lymphoma. Cancers 2021, 13, 3367.Cancers (Basel). 2021 Dec 7;13(24):6151. doi: 10.3390/cancers13246151. Cancers (Basel). 2021. PMID: 34945012 Free PMC article.
Abstract
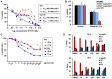
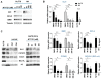
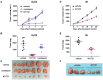
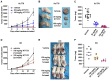
Cutaneous T cell lymphomas (CTCLs) are a heterogeneous group of debilitating, incurable malignancies. Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common subtypes, accounting for ~65% of CTCL cases. Patients with advanced disease have a poor prognosis and low median survival rates of four years. CTCLs develop from malignant skin-homing CD4+ T cells that spread to lymph nodes, blood, bone marrow and viscera in advanced stages. Current treatments options for refractory or advanced CTCL, including chemotherapeutic and biological approaches, rarely lead to durable responses. The exact molecular mechanisms of CTCL pathology remain unclear despite numerous genomic and gene expression profile studies. However, apoptosis resistance is thought to play a major role in the accumulation of malignant T cells. Here we show that NT1721, a synthetic epidithiodiketopiperazine based on a natural product, reduced cell viability at nanomolar concentrations in CTCL cell lines, while largely sparing normal CD4+ cells. Treatment of CTCL cells with NT1721 reduced proliferation and potently induced apoptosis. NT1721 mediated the downregulation of GLI1 transcription factor, which was associated with decreased STAT3 activation and the reduced expression of downstream antiapoptotic proteins (BCL2 and BCL-xL). Importantly, NT1721, which is orally available, reduced tumor growth in two CTCL mouse models significantly better than two clinically used drugs (romidepsin, gemcitabine). Moreover, a combination of NT1721 with gemcitabine reduced the tumor growth significantly better than the single drugs. Taken together, these results suggest that NT1721 may be a promising new agent for the treatment of CTCLs.
Keywords: GLI1; NT1721; STAT3; cutaneous T cell lymphoma (CTCL); epidithiodiketopiperazine.
Conflict of interest statement
D.A.H. and L.E.O. are cofounders of Novonco Therapeutics Inc., Los Angeles, CA, USA. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

