Role of Splicing Regulatory Elements and In Silico Tools Usage in the Identification of Deep Intronic Splicing Variants in Hereditary Breast/Ovarian Cancer Genes
- PMID: 34283047
- PMCID: PMC8268271
- DOI: 10.3390/cancers13133341
Role of Splicing Regulatory Elements and In Silico Tools Usage in the Identification of Deep Intronic Splicing Variants in Hereditary Breast/Ovarian Cancer Genes
Abstract
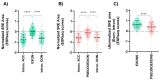
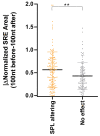
The contribution of deep intronic splice-altering variants to hereditary breast and ovarian cancer (HBOC) is unknown. Current computational in silico tools to predict spliceogenic variants leading to pseudoexons have limited efficiency. We assessed the performance of the SpliceAI tool combined with ESRseq scores to identify spliceogenic deep intronic variants by affecting cryptic sites or splicing regulatory elements (SREs) using literature and experimental datasets. Our results with 233 published deep intronic variants showed that SpliceAI, with a 0.05 threshold, predicts spliceogenic deep intronic variants affecting cryptic splice sites, but is less effective in detecting those affecting SREs. Next, we characterized the SRE profiles using ESRseq, showing that pseudoexons are significantly enriched in SRE-enhancers compared to adjacent intronic regions. Although the combination of SpliceAI with ESRseq scores (considering ∆ESRseq and SRE landscape) showed higher sensitivity, the global performance did not improve because of the higher number of false positives. The combination of both tools was tested in a tumor RNA dataset with 207 intronic variants disrupting splicing, showing a sensitivity of 86%. Following the pipeline, five spliceogenic deep intronic variants were experimentally identified from 33 variants in HBOC genes. Overall, our results provide a framework to detect deep intronic variants disrupting splicing.
Keywords: cryptic splice sites; hereditary breast ovarian cancer; in silico prediction tools; pseudoexons; spliceogenic deep intronic variants; splicing regulatory elements.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Bonache S., Esteban I., Moles-Fernández A., Tenés A., Duran-Lozano L., Montalban G., Bach V., Carrasco E., Gadea N., López-Fernández A., et al. Multigene panel testing beyond BRCA1/2 in breast/ovarian cancer Spanish families and clinical actionability of findings. J. Cancer Res. Clin. Oncol. 2018;144:2495–2513. doi: 10.1007/s00432-018-2763-9. - DOI - PMC - PubMed
-
- Feliubadaló L., López-Fernández A., Pineda M., Díez O., del Valle J., Gutiérrez-Enríquez S., Teulé A., González S., Stjepanovic N., Salinas M., et al. Opportunistic testing of BRCA1, BRCA2 and mismatch repair genes improves the yield of phenotype driven hereditary cancer gene panels. Int. J. Cancer. 2019;145:2682–2691. doi: 10.1002/ijc.32304. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

