Optic Nerve Engraftment of Neural Stem Cells
- PMID: 34283208
- PMCID: PMC8300061
- DOI: 10.1167/iovs.62.9.30
Optic Nerve Engraftment of Neural Stem Cells
Abstract
Purpose: To evaluate the integrative potential of neural stem cells (NSCs) with the visual system and characterize effects on the survival and axonal regeneration of axotomized retinal ganglion cells (RGCs).
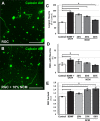
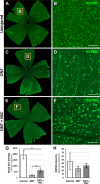
Methods: For in vitro studies, primary, postnatal rat RGCs were directly cocultured with human NSCs or cultured in NSC-conditioned media before their survival and neurite outgrowth were assessed. For in vivo studies, human NSCs were transplanted into the transected rat optic nerve, and immunohistology of the retina and optic nerve was performed to evaluate RGC survival, RGC axon regeneration, and NSC integration with the injured visual system.
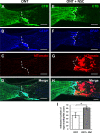
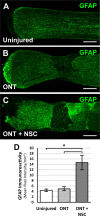
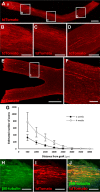
Results: Increased neurite outgrowth was observed in RGCs directly cocultured with NSCs. NSC-conditioned media demonstrated a dose-dependent effect on RGC survival and neurite outgrowth in culture. NSCs grafted into the lesioned optic nerve modestly improved RGC survival following an optic nerve transection (593 ± 164 RGCs/mm2 vs. 199 ± 58 RGCs/mm2; P < 0.01). Additionally, RGC axonal regeneration following an optic nerve transection was modestly enhanced by NSCs transplanted at the lesion site (61.6 ± 8.5 axons vs. 40.3 ± 9.1 axons, P < 0.05). Transplanted NSCs also differentiated into neurons, received synaptic inputs from regenerating RGC axons, and extended axons along the transected optic nerve to incorporate with the visual system.
Conclusions: Human NSCs promote the modest survival and axonal regeneration of axotomized RGCs that is partially mediated by diffusible NSC-derived factors. Additionally, NSCs integrate with the injured optic nerve and have the potential to form neuronal relays to restore retinofugal connections.
Conflict of interest statement
Disclosure:
Figures


References
-
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002; 33: 689–702. - PubMed
-
- Lu P, Jones LL, Snyder EY, Tuszynski MH.. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003; 181: 115–129. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

