Principles of Genomic Newborn Screening Programs: A Systematic Review
- PMID: 34283230
- PMCID: PMC8293022
- DOI: 10.1001/jamanetworkopen.2021.14336
Principles of Genomic Newborn Screening Programs: A Systematic Review
Abstract
Importance: Genomic newborn screening (gNBS) may optimize the health and well-being of children and families. Screening programs are required to be evidence based, acceptable, and beneficial.
Objectives: To identify what has been discovered following the reporting of the first gNBS pilot projects and to provide a summary of key points for the design of gNBS.
Evidence review: A systematic literature review was performed on April 14, 2021, identifying 36 articles that addressed the following questions: (1) what is the interest in and what would be the uptake of gNBS? (2) what diseases and genes should be included? (3) what is the validity and utility of gNBS? and (4) what are the ethical, legal, and social implications? Articles were only included if they generated new evidence; all opinion pieces were excluded.
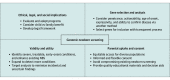
Findings: In the 36 articles included, there was high concordance, except for gene disease inclusion, which was highly variable. Key findings were the need for equitable access, appropriate educational materials, and informed and flexible consent. The process for selecting genes for testing should be transparent and reflect that parents value the certainty of prediction over actionability. Data should be analyzed in a way that minimizes uncertainty and incidental findings. The expansion of traditional newborn screening (tNBS) to identify more life-threatening and treatable diseases needs to be balanced against the complexity of consenting parents of newborns for genomic testing as well as the risk that overall uptake of tNBS may decline. The literature reflected that the right of a child to self-determination should be valued more than the possibility of the whole family benefiting from a newborn genomic test.
Conclusions and relevance: The findings of this systematic review suggest that implementing gNBS will require a nuanced approach. There are gaps in our knowledge, such as the views of diverse populations, the capabilities of health systems, and health economic implications. It will be essential to rigorously evaluate outcomes and ensure programs can evolve to maximize benefit.
Conflict of interest statement
Figures
References
-
- Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. World Health Organization; 1968. Accessed June 15, 2021. http://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf?seq...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous