Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine
- PMID: 34283338
- PMCID: PMC8290394
- DOI: 10.1007/s10238-021-00746-4
Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine
Abstract
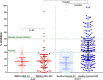
Vaccination against SARS-CoV-2 is considered as the most important preventive strategy against COVID-19, but its efficacy in patients with hematological malignancies is largely unknown. We investigated the development of neutralizing antibodies (NAbs) against SARS-CoV-2 in patients with Waldenstrom Macroglobulinemia (WM), Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin Lymphoma (NHL). After the first dose of the vaccine, on D22, WM/CLL/NHL patients had lower NAb titers compared to controls: the median NAb inhibition titer was 17% (range 0-91%, IQR 8-27%) for WM/CLL/NHL patients versus 32% (range 2-98%, IQR 19-48%) for controls (P < 0.001). Only 8 (14%) patients versus 114 (54%) controls developed NAb titers ≥ 30% on D22 (p < 0.001). Our data indicate that the first dose of both BNT162b2 and AZD1222 leads to lower production of NAbs against SARS-CoV-2 in patients with WM/CLL/NHL compared to controls of similar age and gender and without malignant disease. Even though the response rates were not optimal, vaccination is still considered essential and if possible should be performed before treatment initiation. These patients with suboptimal responses should be considered to be prioritized for booster doses.
Keywords: AZD1222; Antibodies; BNT162b2; COVID-19; Chronic Lymphocytic Leukemia; Non-Hodgkin Lymphoma; SARS-CoV-2; Vaccine; Waldenstrom Macroglobulinemia.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no relevant conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

