Sequential and Switchable Patterning for Studying Cellular Processes under Spatiotemporal Control
- PMID: 34283577
- PMCID: PMC9282641
- DOI: 10.1021/acsami.1c09850
Sequential and Switchable Patterning for Studying Cellular Processes under Spatiotemporal Control
Abstract
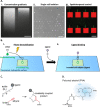
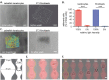
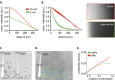
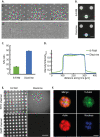
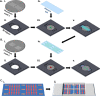
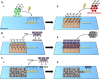
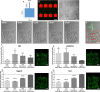
Attachment of adhesive molecules on cell culture surfaces to restrict cell adhesion to defined areas and shapes has been vital for the progress of in vitro research. In currently existing patterning methods, a combination of pattern properties such as stability, precision, specificity, high-throughput outcome, and spatiotemporal control is highly desirable but challenging to achieve. Here, we introduce a versatile and high-throughput covalent photoimmobilization technique, comprising a light-dose-dependent patterning step and a subsequent functionalization of the pattern via click chemistry. This two-step process is feasible on arbitrary surfaces and allows for generation of sustainable patterns and gradients. The method is validated in different biological systems by patterning adhesive ligands on cell-repellent surfaces, thereby constraining the growth and migration of cells to the designated areas. We then implement a sequential photopatterning approach by adding a second switchable patterning step, allowing for spatiotemporal control over two distinct surface patterns. As a proof of concept, we reconstruct the dynamics of the tip/stalk cell switch during angiogenesis. Our results show that the spatiotemporal control provided by our "sequential photopatterning" system is essential for mimicking dynamic biological processes and that our innovative approach has great potential for further applications in cell science.
Keywords: click chemistry; integrin; microcontact printing; photopatterning; surface engineering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Parker K. K.; Brock A. L.; Brangwynne C.; Mannix R. J.; Wang N.; Ostuni E.; Geisse N. A.; Adams J. C.; Whitesides G. M.; Ingber D. E. Directional Control of Lamellipodia Extension by Constraining Cell Shape and Orienting Cell Tractional Forces. FASEB J. 2002, 16, 1195–1204. 10.1096/fj.02-0038com. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

