Reaction Path-Force Matching in Collective Variables: Determining Ab Initio QM/MM Free Energy Profiles by Fitting Mean Force
- PMID: 34283604
- PMCID: PMC9064116
- DOI: 10.1021/acs.jctc.1c00245
Reaction Path-Force Matching in Collective Variables: Determining Ab Initio QM/MM Free Energy Profiles by Fitting Mean Force
Abstract
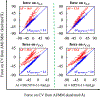
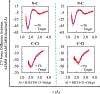
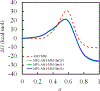
First-principles determination of free energy profiles for condensed-phase chemical reactions is hampered by the daunting costs associated with configurational sampling on ab initio quantum mechanical/molecular mechanical (AI/MM) potential energy surfaces. Here, we report a new method that enables efficient AI/MM free energy simulations through mean force fitting. In this method, a free energy path in collective variables (CVs) is first determined on an efficient reactive aiding potential. Based on the configurations sampled along the free energy path, correcting forces to reproduce the AI/MM forces on the CVs are determined through force matching. The AI/MM free energy profile is then predicted from simulations on the aiding potential in conjunction with the correcting forces. Such cycles of correction-prediction are repeated until convergence is established. As the instantaneous forces on the CVs sampled in equilibrium ensembles along the free energy path are fitted, this procedure faithfully restores the target free energy profile by reproducing the free energy mean forces. Due to its close connection with the reaction path-force matching (RP-FM) framework recently introduced by us, we designate the new method as RP-FM in collective variables (RP-FM-CV). We demonstrate the effectiveness of this method on a type-II solution-phase SN2 reaction, NH3 + CH3Cl (the Menshutkin reaction), simulated with an explicit water solvent. To obtain the AI/MM free energy profiles, we employed the semiempirical AM1/MM Hamiltonian as the base level for determining the string minimum free energy pathway, along which the free energy mean forces are fitted to various target AI/MM levels using the Hartree-Fock (HF) theory, density functional theory (DFT), and the second-order Møller-Plesset perturbation (MP2) theory as the AI method. The forces on the bond-breaking and bond-forming CVs at both the base and target levels are obtained by force transformation from Cartesian to redundant internal coordinates under the Wilson B-matrix formalism, where the linearized FM is facilitated by the use of spline functions. For the Menshutkin reaction tested, our FM treatment greatly reduces the deviations on the CV forces, originally in the range of 12-33 to ∼2 kcal/mol/Å. Comparisons with the experimental and benchmark AI/MM results, tests of the new method under a variety of simulation protocols, and analyses of the solute-solvent radial distribution functions suggest that RP-FM-CV can be used as an efficient, accurate, and robust method for simulating solution-phase chemical reactions.
Figures









Similar articles
-
Doubly Polarized QM/MM with Machine Learning Chaperone Polarizability.J Chem Theory Comput. 2021 Dec 14;17(12):7682-7695. doi: 10.1021/acs.jctc.1c00567. Epub 2021 Nov 1. J Chem Theory Comput. 2021. PMID: 34723536 Free PMC article.
-
Reaction Path Force Matching: A New Strategy of Fitting Specific Reaction Parameters for Semiempirical Methods in Combined QM/MM Simulations.J Chem Theory Comput. 2014 Aug 12;10(8):3038-54. doi: 10.1021/ct4009624. Epub 2014 Jun 6. J Chem Theory Comput. 2014. PMID: 26588275
-
Bridging semiempirical and ab initio QM/MM potentials by Gaussian process regression and its sparse variants for free energy simulation.J Chem Phys. 2023 Aug 7;159(5):054107. doi: 10.1063/5.0156327. J Chem Phys. 2023. PMID: 37530109 Free PMC article.
-
Facilitating ab initio QM/MM free energy simulations by Gaussian process regression with derivative observations.Phys Chem Chem Phys. 2022 Oct 27;24(41):25134-25143. doi: 10.1039/d2cp02820d. Phys Chem Chem Phys. 2022. PMID: 36222412 Free PMC article.
-
Collective Variable-Based Enhanced Sampling: From Human Learning to Machine Learning.J Phys Chem Lett. 2024 Feb 15;15(6):1774-1783. doi: 10.1021/acs.jpclett.3c03542. Epub 2024 Feb 8. J Phys Chem Lett. 2024. PMID: 38329095 Review.
Cited by
-
Machine learning based implicit solvent model for aqueous-solution alanine dipeptide molecular dynamics simulations.RSC Adv. 2023 Feb 3;13(7):4565-4577. doi: 10.1039/d2ra08180f. eCollection 2023 Jan 31. RSC Adv. 2023. PMID: 36760282 Free PMC article.
-
Machine-Learning-Assisted Free Energy Simulation of Solution-Phase and Enzyme Reactions.J Chem Theory Comput. 2021 Sep 14;17(9):5745-5758. doi: 10.1021/acs.jctc.1c00565. Epub 2021 Sep 1. J Chem Theory Comput. 2021. PMID: 34468138 Free PMC article.
-
Development of Range-Corrected Deep Learning Potentials for Fast, Accurate Quantum Mechanical/Molecular Mechanical Simulations of Chemical Reactions in Solution.J Chem Theory Comput. 2021 Nov 9;17(11):6993-7009. doi: 10.1021/acs.jctc.1c00201. Epub 2021 Oct 13. J Chem Theory Comput. 2021. PMID: 34644071 Free PMC article.
-
Doubly Polarized QM/MM with Machine Learning Chaperone Polarizability.J Chem Theory Comput. 2021 Dec 14;17(12):7682-7695. doi: 10.1021/acs.jctc.1c00567. Epub 2021 Nov 1. J Chem Theory Comput. 2021. PMID: 34723536 Free PMC article.
-
Perspectives on Computational Enzyme Modeling: From Mechanisms to Design and Drug Development.ACS Omega. 2024 Feb 8;9(7):7393-7412. doi: 10.1021/acsomega.3c09084. eCollection 2024 Feb 20. ACS Omega. 2024. PMID: 38405524 Free PMC article. Review.
References
-
- Hehre WJ; Radom L; Schleyer P. v. R.; Pople JA Ab Initio Molecular Orbital Theory. John Wiley: New York, 1986.
-
- Kohn W; Sham LJ Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. A 1965, 140, 1133–1138.
-
- Parr RG; Yang W. Density-Functional Theory of Atoms and Molecules Oxford University Press, USA: 1994.
-
- Warshel A; Levitt M. Theoretical Studies of Enzymic Reactions: Dielectric, Electrostatic and Steric Stabilization of the Carbonium Ion in the Reaction of Lysozyme. J. Mol. Biol 1976, 103, 227–249. - PubMed
-
- Field MJ; Bash PA; Karplus M. A Combined Quantum Mechanical and Molecular Mechanical Potential for Molecular Dynamics Simulations. J. Comput. Chem 1990, 11, 700–733.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
