Improved delivery of broadly neutralizing antibodies by nanocapsules suppresses SHIV infection in the CNS of infant rhesus macaques
- PMID: 34283885
- PMCID: PMC8323878
- DOI: 10.1371/journal.ppat.1009738
Improved delivery of broadly neutralizing antibodies by nanocapsules suppresses SHIV infection in the CNS of infant rhesus macaques
Abstract
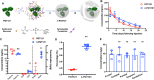
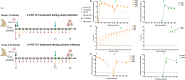
Broadly neutralizing antibodies (bNAbs) directed to HIV-1 have shown promise at suppressing viremia in animal models. However, the use of bNAbs for the central nervous system (CNS) infection is confounded by poor penetration of the blood brain barrier (BBB). Typically, antibody concentrations in the CNS are extremely low; with levels in cerebrospinal fluid (CSF) only 0.1% of blood concentrations. Using a novel nanotechnology platform, which we term nanocapsules, we show effective transportation of the human bNAb PGT121 across the BBB in infant rhesus macaques upon systemic administration up to 1.6% of plasma concentration. We demonstrate that a single dose of PGT121 encased in nanocapsules when delivered at 48h post-infection delays early acute infection with SHIVSF162P3 in infants, with one of four animals demonstrating viral clearance. Importantly, the nanocapsule delivery of PGT121 improves suppression of SHIV infection in the CNS relative to controls.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: I.S.Y.C. has a financial interest in CSL Behring and Calimmune Inc. J.W. has a financial interest in Vivibaba and The Regents have licensed intellectual property invented by J.W. to Vivibaba. Y.L. has a financial interest in Vivibaba and The Regents have licensed intellectual property invented by Y.L. to Vivibaba. No funding was provided by these companies to support this work.
Figures




References
-
- Grant I, Atkinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1987;107(6):828–36. doi: 10.7326/0003-4819-107-6-828 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

