Computational analysis of TMPRSS2 expression in normal and SARS-CoV-2-infected human tissues
- PMID: 34284028
- PMCID: PMC8285370
- DOI: 10.1016/j.cbi.2021.109583
Computational analysis of TMPRSS2 expression in normal and SARS-CoV-2-infected human tissues
Abstract
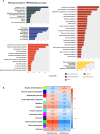
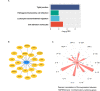
The transmembrane serine protease 2 (TMPRSS2) is a key molecule for SARS-CoV-2 invading human host cells. To provide insights into SARS-CoV-2 infection of various human tissues and understand the potential mechanism of SARS-CoV-2 infection, we investigated TMPRSS2 expression in various normal human tissues and SARS-CoV-2-infected human tissues. Using publicly available datasets, we performed computational analyses of TMPRSS2 expression levels in 30 normal human tissues, and compared them between males and females and between younger (ages ≤ 49 years) and older (ages > 49 years) populations in these tissues. We found that TMPRSS2 expression levels were the highest in the prostate, stomach, pancreas, lungs, small intestine, and salivary gland. The TMPRSS2 protein had relatively high expression levels in the parathyroid gland, stomach, small intestine, pancreas, kidneys, seminal vesicle, epididymis, and prostate. However, TMPRSS2 expression levels were not significantly different between females and males or between younger and older populations in these tissues. The pathways enriched in TMPRSS2-upregulated pan-tissue were mainly involved in immune, metabolism, cell growth and proliferation, stromal signatures, and cancer and other diseases. Many cytokine genes displayed positive expression correlations with TMPRSS2 in pan-tissue, including TNF-α, IL-1, IL-2, IL-4, IL-7, IL-8, IL-12, IL-18, IFN-α, MCP-1, G-CSF, and IP-10. We further analyzed TMPRSS2 expression levels in nasopharyngeal swabs from SARS-CoV-2-infected patients. TMPRSS2 expression levels showed no significant difference between males and females or between younger and older patients. However, they were significantly lower in SARS-CoV-2-infected than in healthy individuals and patients with other viral acute respiratory illnesses. Interestingly, TMPRSS2 expression levels were positively correlated with the enrichment levels of four immune signatures (B cells, CD8+ T cells, NK cells, and interferon response) in SARS-CoV-2-infected patients but likely to be negatively correlated with them in the normal lung tissue. Our data may provide insights into the mechanism of SARS-CoV-2 infection.
Keywords: Gene co-expression network; Gene expression profiles; Immune signatures; Pathway and gene ontology; SARS-CoV-2; Transmembrane serine protease 2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- COVID-19 Dashboard Johns hopkins university. 2020. https://coronavirus.jhu.edu/map.html
-
- Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pöhlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

