The reliability of saliva for the detection of SARS-CoV-2 in symptomatic and asymptomatic patients: Insights on the diagnostic performance and utility for COVID-19 screening
- PMID: 34284319
- PMCID: PMC8180088
- DOI: 10.1016/j.diagmicrobio.2021.115450
The reliability of saliva for the detection of SARS-CoV-2 in symptomatic and asymptomatic patients: Insights on the diagnostic performance and utility for COVID-19 screening
Abstract
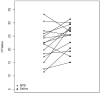
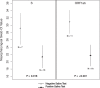
Current literature has focused on testing saliva in symptomatic patients, and little information is available regarding saliva performance in asymptomatic severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection. We compared paired saliva and nasopharyngeal swabs (NPS) collected from 33 symptomatic and 12 asymptomatic known SARS-CoV-2-positive patients. Saliva had an overall sensitivity of 59%, a specificity of 95%, and a negative predictive value of 98%. Saliva demonstrated higher sensitivity in symptomatic (80%) vs. asymptomatic individuals (36%) (P = 0.006), and in high-risk (symptomatic, febrile and/or with comorbidities) (82%) vs. low-risk (asymptomatic, afebrile, and no comorbidities) (22%) patients (P = 0.0002). Cycle threshold (Ct) values in NPS specimens were higher in saliva-negative vs. saliva-positive cases (P = 0.02 and <0.001). Overall, these findings show that despite saliva's low sensitivity in asymptomatic SARS-CoV-2 infections, it can detect infections with lower Ct values and a potentially higher chance of viral transmission. Additional studies are warranted to fully evaluate saliva as a screening test for coronavirus disease-2019.
Keywords: Asymptomatic; COVID-19; Nasopharyngeal swab; SARS-CoV-2; Saliva; Screening.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
-
- Becker D, Sandoval E, Amin A, De Hoff P, Diets A, Leonetti N, et al. Saliva is less sensitive than nasopharyngeal swabs for COVID-19 detection in the community setting. 2020. medRxiv:2020.2005.2011.20092338. doi:10.1101/2020.05.11.20092338
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous