Regression of taxane-related cystoid macular edema after topical dorzolamide treatment: two case reports
- PMID: 34284818
- PMCID: PMC8293476
- DOI: 10.1186/s13256-021-02954-8
Regression of taxane-related cystoid macular edema after topical dorzolamide treatment: two case reports
Abstract
Background: Cystoid macular edema is a rare, vision-threatening side effect of the taxane family of anticancer agents. There is no established treatment or standard treatment protocol for taxane-related cystoid macular edema. Here, we report two cases of taxane-related cystoid macular edema that were treated with topical dorzolamide.
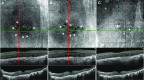
Case presentation: In case 1, a 72-year-old Japanese woman with bilateral geographic choroiditis reported for a follow-up visit with a complaint of blurred vision in both eyes for 2 months after starting nanoparticle albumin-bound paclitaxel chemotherapy for multiple metastases of her breast cancer. Her best-corrected visual acuity had dropped from 1.2 to 0.9 in the right eye and from 1.0 to 0.4 in the left eye. Fundus examination showed no newly active geographic choroiditis lesion, but optical coherence tomography exhibited cystoid macular edema. We suspected taxane-related cystoid macular edema and terminated nanoparticle albumin-bound paclitaxel, and started topical dorzolamide treatment. Cystoid macular edema nearly resolved within 6 weeks in the right eye and within 10 weeks in the left eye after starting topical dorzolamide treatment. The resolution of cystoid macular edema without leaving a chorioretinal scar after discontinuation of paclitaxel confirmed our initial diagnosis of taxane-related cystoid macular edema. A few inconspicuous cystoid spaces persisted at the parafovea for a year after dorzolamide treatment ended, but regressed after restarting dorzolamide treatment without any side effects. Best-corrected visual acuity improved to 1.2 in the right eye and 1.0 in the left eye. In case 2, a 70-year-old Japanese man, who received nanoparticle albumin-bound paclitaxel for pancreatic cancer with multiple metastases, developed bilateral cystoid macular edema. Best-corrected visual acuity was 0.3 bilaterally. Cystoid macular edema resolved within 5 weeks after stopping nanoparticle albumin-bound paclitaxel and starting topical dorzolamide treatment confirming the diagnosis of taxane-related cystoid macular edema. Nine weeks later, best-corrected visual acuity improved to 0.8 in the right eye and 1.0 in the left eye.
Conclusions: Cystoid macular edema in each case resolved within a few months without any side effects using topical dorzolamide and terminating taxane-based chemotherapy. Topical dorzolamide appears to be a safe and effective treatment option for patients with taxane-related cystoid macular edema whose quality of life is threatened by visual disturbances.
Keywords: Cystoid macular edema; Dorzolamide; Nab-paclitaxel; Optical coherence tomography; Taxane.
© 2021. The Author(s).
Conflict of interest statement
The authors have no financial or nonfinancial competing interests to declare.
Figures




Similar articles
-
Unraveling the Mystery of Taxol-Induced Cystoid Macular Oedema: Case Report and Literature Review.Rom J Ophthalmol. 2025 Jan-Mar;69(1):3-9. doi: 10.22336/rjo.2025.02. Rom J Ophthalmol. 2025. PMID: 40330966 Free PMC article. Review.
-
POSSIBLE EFFICACY OF TOPICAL DORZOLAMIDE IN THE TREATMENT OF PACLITAXEL-RELATED CYSTOID MACULAR EDEMA.Retin Cases Brief Rep. 2018 Winter;12(1):75-79. doi: 10.1097/ICB.0000000000000433. Retin Cases Brief Rep. 2018. PMID: 27749791
-
Efficacy of dorzolamide hydrochloride in the management of chronic cystoid macular edema in patients with retinitis pigmentosa.Retina. 1997;17(3):222-31. doi: 10.1097/00006982-199705000-00009. Retina. 1997. PMID: 9196934 Clinical Trial.
-
Case Report: Macula Structural and Functional Assessment in Nab-Paclitaxel-Related Cystoid Macular Edema.Ophthalmic Surg Lasers Imaging Retina. 2024 Jul;55(7):408-411. doi: 10.3928/23258160-20240227-01. Epub 2024 Mar 1. Ophthalmic Surg Lasers Imaging Retina. 2024. PMID: 38531022
-
Nab-Paclitaxel related cystoid macular edema.Clin Ter. 2022 Jul-Aug;173(4):377-383. doi: 10.7417/CT.2022.2449. Clin Ter. 2022. PMID: 35857057 Review.
Cited by
-
Unraveling the Mystery of Taxol-Induced Cystoid Macular Oedema: Case Report and Literature Review.Rom J Ophthalmol. 2025 Jan-Mar;69(1):3-9. doi: 10.22336/rjo.2025.02. Rom J Ophthalmol. 2025. PMID: 40330966 Free PMC article. Review.
-
Bilateral macular edema secondary to nab-paclitaxel therapy for breast cancer.Int J Ophthalmol. 2024 Oct 18;17(10):1963-1966. doi: 10.18240/ijo.2024.10.26. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39430033 Free PMC article. No abstract available.
-
Efficacy of topical carbonic anhydrase inhibitors in treating taxane drug-induced cystoid macular edema: A case report.Medicine (Baltimore). 2025 Jan 3;104(1):e40958. doi: 10.1097/MD.0000000000040958. Medicine (Baltimore). 2025. PMID: 40184125 Free PMC article.
-
Intravitreal Ranibizumab Had Limited Effect on Cystoid Macular Edema Due to Albumin-Bound Paclitaxel: A Case Report and Literature Review.Front Oncol. 2021 Dec 13;11:773540. doi: 10.3389/fonc.2021.773540. eCollection 2021. Front Oncol. 2021. PMID: 34966680 Free PMC article.
-
The Role of Nanoparticles in Therapy of Real-World Patients with Pancreatic Cancer: A Scoping Review.Cancers (Basel). 2025 May 21;17(10):1726. doi: 10.3390/cancers17101726. Cancers (Basel). 2025. PMID: 40427222 Free PMC article. Review.
References
-
- ABRAXANE® [package insert]. Summit, NJ: Celgene Corporation. 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

