PTD-mediated delivery of α-globin chain into Κ-562 erythroleukemia cells and α-thalassemic (HBH) patients' RBCs ex vivo in the frame of Protein Replacement Therapy
- PMID: 34284828
- PMCID: PMC8290593
- DOI: 10.1186/s40709-021-00148-3
PTD-mediated delivery of α-globin chain into Κ-562 erythroleukemia cells and α-thalassemic (HBH) patients' RBCs ex vivo in the frame of Protein Replacement Therapy
Abstract
Background: α-Thalassemia, a congenital hemoglobinopathy, is characterized by deficiency and/or reduced levels of α-globin chains in serious forms of α-thalassemia (HbH disease/Hb Bart's). This research work deals with a Protein Replacement Therapy approach in order to manage α-thalassemia manifestations, caused by the excess of β-globin chain into HbH RBCs. The main goal was to produce the recombinant human α-globin chain in fusion with TAT, a Protein Transduction Domain, to ex vivo deliver it into HbH patients RBCs, to replace the endogenous missing α-globin chain.
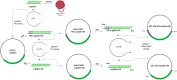
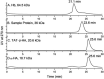
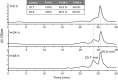
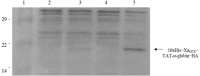
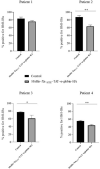
Results: Cloning of the α-globin coding sequence, fused to the nucleotide sequence of TAT peptide was conducted and the human recombinant fusion proteins, 10xHis-XaSITE-α-globin-HA and 10xHis-XaSITE-TAT-α-globin-HA were produced. The ability of human recombinant 10xHis-XaSITE-α-globin-HA to interact in vitro with the previously produced 10xHis-XaSITE-TAT-β-globin-HA and form α-/β-globin heterodimers, was assessed and confirmed by size exclusion chromatography. The recombinant 10xHis-XaSITE-TAT-α-globin-HA was successfully delivered into human proerythroid K-562 cells, during the preliminary transduction evaluation experiments. Finally, the recombinant, TAT-fused α-globin was successfully transduced into RBCs, derived from HbH patients and reduced the formation of HbH-Inclusion Bodies, known to contain harmful β4-globin chain tetramers.
Conclusions: Our data confirm the successful ex vivo transduction of recombinant α-globin chains in HbH RBCs to replace the missing a-globin chain and reduce the HbH-inclusion bodies, seen in α-thalassemias. These findings broaden the possibility of applying a Protein Replacement Therapy approach to module sever forms of α-thalassemia, using recombinant α-globin chains, through PTD technology.
Keywords: HbH thalassemic patients’ RBCs; Intracellular transduction via PTD; LC − MS/MS analysis; Size exclusion chromatography; TAT-α-globin.
© 2021. The Author(s).
Conflict of interest statement
All authors declare no conflict of interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

