Vaccinating Australia: How long will it take?
- PMID: 34284875
- PMCID: PMC8285754
- DOI: 10.1016/j.vaccine.2021.07.006
Vaccinating Australia: How long will it take?
Abstract
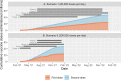
The Australian Government began to roll out the national COVID-19 vaccination program in late February 2021, with the initial aim to vaccinate the Australian adult population by the end of October 2021. The task of vaccinating some 20 million people presents considerable logistic challenges, but a rapid rollout is essential to allow for the reopening of borders and is especially urgent as new more transmissible variants arise. Here, we run a series of projections to estimate how long it will take to vaccinate the Australian population under different assumptions about the rate of vaccine administration, the schedule for vaccine eligibility and prevalence of vaccine hesitancy. Our analysis highlights the number of vaccine doses that can be administered per day as the key factor determining the duration of the vaccine rollout. A rate of 200,000 doses per day would achieve 90% population coverage by the end of 2021; 80,000 doses a day would see the rollout extended until mid-2023. Vaccine hesitancy has the potential to greatly slow down the rollout and becomes the main limiting factor when the supply of vaccine doses is high. Speed is of the essence when it comes vaccinating populations against COVID-19: a rapid rollout will minimise the risk of sporadic and costly lockdowns and the potential for small, local clusters getting out of control and sparking new epidemic waves. In order to achieve rapid population coverage, the Australian government must ramp up vaccine administration to at least 200,000 doses per day as quickly as possible, while also promoting vaccine willingness in the community through clear public health messaging, especially to known hesitant demographics.
Keywords: Australia; COVID-19; Vaccination.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Raina MacIntyre has been on an advisory board for COVID-19 vaccine for Seqirus and consulted or done speaking engagements on COVID-19 vaccines for Janssen and Astra Zeneca].
Figures
References
-
- Doorstop interview on 31 January 2021 Australian Government Department of Health. Published online January 2021. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/doorstop-....
-
- Australia’s COVID-19 vaccine national roll-out strategy. Published online December 2020. https://www.health.gov.au/sites/default/files/documents/2021/01/australi....
-
- Australian Government Department of Health. ATAGI statement on AstraZeneca vaccine in response to new vaccine safety concerns. Published online 8 April 2021. https://www.health.gov.au/news/atagi-statement-on-astrazeneca-vaccine-in....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical