T-Cell Expression and Release of Kidney Injury Molecule-1 in Response to Glucose Variations Initiates Kidney Injury in Early Diabetes
- PMID: 34285121
- PMCID: PMC8385614
- DOI: 10.2337/db20-1081
T-Cell Expression and Release of Kidney Injury Molecule-1 in Response to Glucose Variations Initiates Kidney Injury in Early Diabetes
Abstract
Half of the mortality in diabetes is seen in individuals <50 years of age and commonly predicted by the early onset of diabetic kidney disease (DKD). In type 1 diabetes, increased urinary albumin-to-creatinine ratio (uACR) during adolescence defines this risk, but the pathological factors responsible remain unknown. We postulated that early in diabetes, glucose variations contribute to kidney injury molecule-1 (KIM-1) release from circulating T cells, elevating uACR and DKD risk. DKD risk was assigned in youth with type 1 diabetes (n = 100; 20.0 ± 2.8 years; males/females, 54:46; HbA1c 66.1 [12.3] mmol/mol; diabetes duration 10.7 ± 5.2 years; and BMI 24.5 [5.3] kg/m2) and 10-year historical uACR, HbA1c, and random blood glucose concentrations collected retrospectively. Glucose fluctuations in the absence of diabetes were also compared with streptozotocin diabetes in apolipoprotein E -/- mice. Kidney biopsies were used to examine infiltration of KIM-1-expressing T cells in DKD and compared with other chronic kidney disease. Individuals at high risk for DKD had persistent elevations in uACR defined by area under the curve (AUC; uACRAUC0-10yrs, 29.7 ± 8.8 vs. 4.5 ± 0.5; P < 0.01 vs. low risk) and early kidney dysfunction, including ∼8.3 mL/min/1.73 m2 higher estimated glomerular filtration rates (modified Schwartz equation; Padj < 0.031 vs. low risk) and plasma KIM-1 concentrations (∼15% higher vs. low risk; P < 0.034). High-risk individuals had greater glycemic variability and increased peripheral blood T-cell KIM-1 expression, particularly on CD8+ T cells. These findings were confirmed in a murine model of glycemic variability both in the presence and absence of diabetes. KIM-1+ T cells were also infiltrating kidney biopsies from individuals with DKD. Healthy primary human proximal tubule epithelial cells exposed to plasma from high-risk youth with diabetes showed elevated collagen IV and sodium-glucose cotransporter 2 expression, alleviated with KIM-1 blockade. Taken together, these studies suggest that glycemic variations confer risk for DKD in diabetes via increased CD8+ T-cell production of KIM-1.
© 2021 by the American Diabetes Association.
Figures

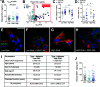

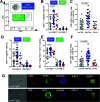
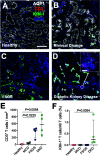
Comment in
-
Glycemic Variability and KIM-1-Induced Inflammation in the Diabetic Kidney.Diabetes. 2021 Aug;70(8):1617-1619. doi: 10.2337/dbi21-0021. Epub 2021 Jul 20. Diabetes. 2021. PMID: 34285120 Free PMC article. No abstract available.
References
-
- Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia 2016;59:1177–1185 - PubMed
-
- Gregg EW, Cheng YJ, Srinivasan M, et al. . Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018;391:2430–2440 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

