Immunogenicity and Safety of AS03-adjuvanted H5N1 Influenza Vaccine in Children 6-35 Months of Age: Results From a Phase 2, Randomized, Observer-blind, Multicenter, Dose-ranging Study
- PMID: 34285165
- PMCID: PMC8357047
- DOI: 10.1097/INF.0000000000003247
Immunogenicity and Safety of AS03-adjuvanted H5N1 Influenza Vaccine in Children 6-35 Months of Age: Results From a Phase 2, Randomized, Observer-blind, Multicenter, Dose-ranging Study
Abstract
Background: This phase 2 observer-blind, randomized, multicenter, dose-ranging study evaluated immunogenicity and safety of different formulations of an AS03-adjuvanted H5N1 influenza vaccine in children 6-35 months of age.
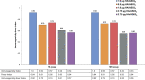
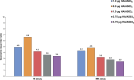
Methods: One hundred eighty-five children randomized into 5 groups [1.9 µg hemagglutinin (HA)/AS03B, 0.9 µg HA/AS03C, 1.9 µg HA/AS03C, 3.75 µg HA/AS03C or 3.75 µg HA/AS03D] were to receive 2 doses administered 21 days apart (primary vaccination). AS03 was classified by amount of DL-α-tocopherol, with AS03B the highest amount. One year later, all subjects were to receive unadjuvanted 3.75 µg HA as antigen challenge. Immunogenicity was assessed 21 days after primary vaccination (day 42) and 7 days after antigen challenge (day 392). Immunogenicity-fever index, based on hemagglutination inhibition and microneutralization antibody titers at day 42 and fever 7 days after each vaccination, was used to guide the selection of an acceptable formulation.
Results: After primary vaccination, formulations elicited strong homologous immune responses with all subjects' hemagglutination inhibition titers ≥1:40 post-vaccination. Immunogenicity-fever index based on hemagglutination inhibition and microneutralization assays showed that 1.9 µg HA/AS03B ranked the highest. Antibody levels persisted >4 times above baseline 12 months after primary vaccination with all formulations (day 385). Antibodies increased >4-fold after antigen challenge (day 392/day 385) with 1.9 µg HA/AS03B, 0.9 µg HA/AS03C and 1.9 µg HA/AS03C formulations. Overall per subject, the incidence of fever ranged from 28.6% (3.75 µg HA/AS03D) to 60.5% (1.9 µg HA/AS03B).
Conclusions: All formulations were highly immunogenic and demonstrated acceptable safety profiles, with the 1.9 µg HA/AS03B providing the most favorable balance of immunogenicity versus reactogenicity for use in children 6-35 months of age.
Trial registration: ClinicalTrials.gov NCT02719743.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
J.H.K., M.D., J.D., D.F. and B.S. are employees of the GSK group of companies. W.W., D.W.V., B.I. and A.S. were employees of the GSK group of companies during the conduct of the study. J.H.K., B.S., M.D., J.D., W.W. and A.S. hold shares from the GSK group of companies. T.P., N.-C.C., K.S. and L.-M.H. received funds from the GSK group of companies through their institution for the conduct of the study. L.-M.H. received personal fees from the GSK group of companies for educational lectures. J.H.K., M.D., T.P., N.-C.C., K.S., L.-M.H., J.D., D.F., B.S., W.W., D.W.V., B.I. and A.S. declare no other financial and nonfinancial relationships and activities. K.-P.H., P.-Y.C. and C.-H.C. declare no financial and nonfinancial relationships and activities and no conflicts of interest.
Figures

References
-
- World Health Organization. H5N1 highly pathogenic avian influenza: timeline of major events. 2014. Available at: http://www.who.int/influenza/human_animal_interface/H5N1_avian_influenza.... Accessed July 1, 2019.
-
- Chu DW, Hwang SJ, Lim FS, et al. ; H5N1 Flu Study Group for Hong Kong, Singapore, Taiwan and Thailand. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009;27:7428–7435. - PubMed
-
- Díez-Domingo J, Garcés-Sanchez M, Baldó JM, et al. . Immunogenicity and Safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase II, randomized, open, controlled study. Pediatr Infect Dis J. 2010;29:e35–e46. - PubMed

