Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury
- PMID: 34285231
- PMCID: PMC8292346
- DOI: 10.1038/s41467-021-24712-6
Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury
Abstract
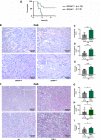
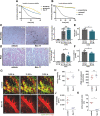
Acute kidney injury (AKI) is morphologically characterized by a synchronized plasma membrane rupture of cells in a specific section of a nephron, referred to as acute tubular necrosis (ATN). Whereas the involvement of necroptosis is well characterized, genetic evidence supporting the contribution of ferroptosis is lacking. Here, we demonstrate that the loss of ferroptosis suppressor protein 1 (Fsp1) or the targeted manipulation of the active center of the selenoprotein glutathione peroxidase 4 (Gpx4cys/-) sensitize kidneys to tubular ferroptosis, resulting in a unique morphological pattern of tubular necrosis. Given the unmet medical need to clinically inhibit AKI, we generated a combined small molecule inhibitor (Nec-1f) that simultaneously targets receptor interacting protein kinase 1 (RIPK1) and ferroptosis in cell lines, in freshly isolated primary kidney tubules and in mouse models of cardiac transplantation and of AKI and improved survival in models of ischemia-reperfusion injury. Based on genetic and pharmacological evidence, we conclude that GPX4 dysfunction hypersensitizes mice to ATN during AKI. Additionally, we introduce Nec-1f, a solid inhibitor of RIPK1 and weak inhibitor of ferroptosis.
© 2021. The Author(s).
Conflict of interest statement
A. L., C. S, and M. G issued a patent for Nec-1f with the number 20160943.5. M. C. is co-founder and shareholder of ROSCUE Therapeutics GmbH. Apart from this, all authors declare no conflict of interest regarding any of the presented data in this paper.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

