Visual instrumental learning in blindsight monkeys
- PMID: 34285293
- PMCID: PMC8292513
- DOI: 10.1038/s41598-021-94192-7
Visual instrumental learning in blindsight monkeys
Abstract
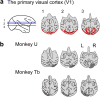
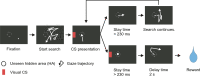
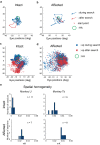
Blindsight is the residual visuo-motor ability without subjective awareness observed after lesions of the primary visual cortex (V1). Various visual functions are retained, however, instrumental visual associative learning remains to be investigated. Here we examined the secondary reinforcing properties of visual cues presented to the hemianopic field of macaque monkeys with unilateral V1 lesions. Our aim was to test the potential role of visual pathways bypassing V1 in reinforcing visual instrumental learning. When learning the location of a hidden area in an oculomotor search task, conditioned visual cues presented to the lesion-affected hemifield operated as an effective secondary reinforcer. We noted that not only the hidden area location, but also the vector of the saccade entering the target area was reinforced. Importantly, when the visual reinforcement signal was presented in the lesion-affected field, the monkeys continued searching, as opposed to stopping when the cue was presented in the intact field. This suggests the monkeys were less confident that the target location had been discovered when the reinforcement cue was presented in the affected field. These results indicate that the visual signals mediated by the residual visual pathways after V1 lesions can access fundamental reinforcement mechanisms but with impaired visual awareness.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures