The human connectome in Alzheimer disease - relationship to biomarkers and genetics
- PMID: 34285392
- PMCID: PMC8403643
- DOI: 10.1038/s41582-021-00529-1
The human connectome in Alzheimer disease - relationship to biomarkers and genetics
Erratum in
-
Author Correction: The human connectome in Alzheimer disease - relationship to biomarkers and genetics.Nat Rev Neurol. 2021 Sep;17(9):592. doi: 10.1038/s41582-021-00554-0. Nat Rev Neurol. 2021. PMID: 34381201 No abstract available.
Abstract
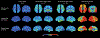
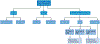
The pathology of Alzheimer disease (AD) damages structural and functional brain networks, resulting in cognitive impairment. The results of recent connectomics studies have now linked changes in structural and functional network organization in AD to the patterns of amyloid-β and tau accumulation and spread, providing insights into the neurobiological mechanisms of the disease. In addition, the detection of gene-related connectome changes might aid in the early diagnosis of AD and facilitate the development of personalized therapeutic strategies that are effective at earlier stages of the disease spectrum. In this article, we review studies of the associations between connectome changes and amyloid-β and tau pathologies as well as molecular genetics in different subtypes and stages of AD. We also highlight the utility of connectome-derived computational models for replicating empirical findings and for tracking and predicting the progression of biomarker-indicated AD pathophysiology.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



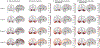

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

