Evaluation of Intraocular Lens Rotational Stability in a Multicenter Clinical Trial
- PMID: 34285467
- PMCID: PMC8286729
- DOI: 10.2147/OPTH.S309214
Evaluation of Intraocular Lens Rotational Stability in a Multicenter Clinical Trial
Abstract
Purpose: To evaluate the postoperative rotational stability of two prototype intraocular lens (IOL) designs (subsequently termed version 1 and version 2).
Patients and methods: A prospective, multicenter, randomized, paired-eye, 6-month study evaluated the version 1 and version 2 IOLs. Results were compared with a control IOL (TECNIS® toric 1-piece monofocal IOL) evaluated in a separate, similarly designed study. Participants aged ≥22 years and scheduled to undergo bilateral cataract extraction were randomly assigned 1:1 to receive the version 1 or version 2 IOL in the first operative eye; the alternate test IOL was then implanted in the second operative eye.
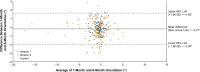
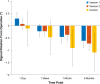
Results: Mean absolute IOL rotation at postoperative week 1 was the primary effectiveness end point. Additional end points included the percentage of eyes with postoperative IOL rotation >5°/>10°, direction of lens rotation, surgeon-reported ease of IOL handling during implantation, and safety. At postoperative week 1, mean (±standard deviation) absolute IOL rotation was significantly lower for both version 1 and version 2 versus control (0.88° [±0.94] and 0.71° [±0.69] vs 2.24° [±3.21], respectively; both P < 0.001). For both study lenses, absolute rotation was <5° for all eyes at postoperative week 1, and no cases of rotation >10° were observed at any postoperative time point. From postoperative week 1 onward, version 2 had a statistically significant clockwise bias in the direction of rotation (P = 0.03); similar findings were observed for version 1. Surgeons reported acceptable ease of IOL handling during implantation for both version 1 and version 2. No device-related adverse events were reported.
Conclusion: Both the version 1 and version 2 IOLs, each with frosted, squared haptics, demonstrated improved postoperative rotational stability compared with a control lens without frosted haptics. Because version 2 had the same overall geometry as the current TECNIS toric IOL, this design was selected for commercialization.
Trial registration: German Clinical Trials Register, DRKS00015287.
Keywords: IOL rotation; astigmatism; cataract; surgery; toric IOL.
© 2021 Vukich et al.
Conflict of interest statement
The authors have made the following disclosures: J. A. V.: Personal fees and nonfinancial support from Johnson & Johnson Surgical Vision during the conduct of the study; grants and personal fees from Johnson & Johnson Surgical Vision outside the submitted work. R. E. A.: Grants from Johnson & Johnson Surgical Vision during the conduct of the study. B. J. K. S.: Personal fees and nonfinancial support from Johnson & Johnson Surgical Vision during the conduct of the study; personal fees, nonfinancial support, and other (owns shares) from Johnson & Johnson Surgical Vision outside the submitted work. D. P. J.: Employee of Johnson & Johnson Surgical Vision at the time of manuscript preparation. P. J. S.: Personal fees from Johnson & Johnson Surgical Vision during the conduct of the study. J. F. B.: Grants and personal fees from Johnson & Johnson Surgical Vision outside the submitted work. K. L. W.: Honoraria from and research services for Johnson & Johnson Surgical Vision during the conduct of the study; personal fees from and research support and/or consulting relationship with Alcon, BVI, Johnson & Johnson, Rayner, Bausch Health, and Zeiss, outside the submitted work.
Figures





Similar articles
-
Reproducibility of the Magnitude of Lens Rotation Following Implantation of a Toric Intraocular Lens with Modified Haptics.Clin Ophthalmol. 2022 Sep 29;16:3213-3224. doi: 10.2147/OPTH.S373976. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36199805 Free PMC article. Clinical Trial.
-
Post-Market Evaluation of Rotational Stability and Visual Performance of a New Toric Intraocular Lens with Frosted Haptics.Clin Ophthalmol. 2022 Dec 10;16:4055-4064. doi: 10.2147/OPTH.S389304. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36532824 Free PMC article.
-
Clinical outcomes of TECNIS toric intraocular lens implantation after cataract removal in patients with corneal astigmatism.Ophthalmology. 2015 Jan;122(1):39-47. doi: 10.1016/j.ophtha.2014.06.027. Epub 2014 Nov 18. Ophthalmology. 2015. PMID: 25444352 Clinical Trial.
-
Early-stage clinical outcomes and rotational stability of TECNIS toric intraocular lens implantation in cataract cases with long axial length.BMC Ophthalmol. 2020 May 25;20(1):204. doi: 10.1186/s12886-020-01465-2. BMC Ophthalmol. 2020. PMID: 32450828 Free PMC article.
-
Comparing Rotational Stability Over Time Between Four Monofocal Toric Intraocular Lenses.Clin Ophthalmol. 2025 Apr 23;19:1345-1355. doi: 10.2147/OPTH.S522728. eCollection 2025. Clin Ophthalmol. 2025. PMID: 40297671 Free PMC article.
Cited by
-
Reproducibility of the Magnitude of Lens Rotation Following Implantation of a Toric Intraocular Lens with Modified Haptics.Clin Ophthalmol. 2022 Sep 29;16:3213-3224. doi: 10.2147/OPTH.S373976. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36199805 Free PMC article. Clinical Trial.
-
Toric intraocular lenses: Evidence-based use.Clin Exp Ophthalmol. 2022 Jul;50(5):481-489. doi: 10.1111/ceo.14106. Epub 2022 May 29. Clin Exp Ophthalmol. 2022. PMID: 35584257 Free PMC article. Review.
-
Rotational Stability and Subjective Results of Tecnis Eyhance Toric II Intraocular Lens.Clin Ophthalmol. 2025 Aug 28;19:3011-3017. doi: 10.2147/OPTH.S515517. eCollection 2025. Clin Ophthalmol. 2025. PMID: 40905026 Free PMC article.
-
Repositioning Rates of Toric IOLs Implanted in Cataract Surgery Patients: A Retrospective Chart Review.Clin Ophthalmol. 2023 Dec 23;17:4001-4007. doi: 10.2147/OPTH.S441524. eCollection 2023. Clin Ophthalmol. 2023. PMID: 38152615 Free PMC article.
-
Post-Market Evaluation of Rotational Stability and Visual Performance of a New Toric Intraocular Lens with Frosted Haptics.Clin Ophthalmol. 2022 Dec 10;16:4055-4064. doi: 10.2147/OPTH.S389304. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36532824 Free PMC article.
References
-
- Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2016;12:275–286. - PubMed
-
- The TECNIS® Toric 1-Piece (IOL). Santa Ana, CA: Johnson & Johnson Vision, Inc; 2020. Available from: https://www.precisionlens.net/wp-content/uploads/JJ-Tecnis-Toric-DFU.pdf. Accessed June29, 2020.
LinkOut - more resources
Full Text Sources

