Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications
- PMID: 34285534
- PMCID: PMC8286087
- DOI: 10.2147/CCID.S313429
Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications
Abstract
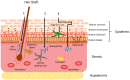
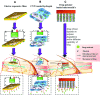
Nanocarrier technology has been effectively applied to the development of drug delivery systems to overcome the limitations of traditional preparation. Its application has been extended to various pharmaceutical fields from injection preparation to oral preparation and external preparation, and now it has appeared in the field of cosmetics for beauty applications. The widespread influence of nanocarrier in the cosmetics industry is due to the fact that nanocarrier can effectively promote the percutaneous penetration and significantly increase skin retention of active components in functional cosmetics. Meanwhile, nanocarrier can effectively improve the water dispersion of insoluble active cosmetic ingredients, enhance the stability of efficacy components and achieve the codelivery of diverse cosmetics active ingredients. In this review, we summarized the current progress of nanocarrier technology in the functional cosmetics, including the types and the routes of dermal/transdermal drug delivery nanocarriers used in the functional cosmetics, the mechanism of nanocarriers promoting the percutaneous penetration of active cosmetic ingredients, the application and efficacy evaluation of different active cosmetic ingredients in nanocarriers and discussing the potential risks to human. This will provide a useful reference for the further development of nanocarriers in the field of functional cosmetics.
Keywords: active cosmetic ingredients; codelivery nanocarriers; efficacy evaluation; functional cosmetics; nanocarrier technology; skin penetration and positioning.
© 2021 Zhou et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

