Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism
- PMID: 34285967
- PMCID: PMC8275291
- DOI: 10.21769/BioProtoc.2850
Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism
Abstract
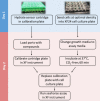
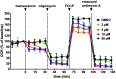
Mammalian cells generate ATP by mitochondrial (oxidative phosphorylation) and non-mitochondrial (glycolysis) metabolism. Cancer cells are known to reprogram their metabolism using different strategies to meet energetic and anabolic needs ( Koppenol et al., 2011 ; Zheng, 2012). Additionally, each cancer tissue has its own individual metabolic features. Mitochondria not only play a key role in energy metabolism but also in cell cycle regulation of cells. Therefore, mitochondria have emerged as a potential target for anticancer therapy since they are structurally and functionally different from their non-cancerous counterparts (D'Souza et al., 2011). We detail a protocol for measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) measurements in living cells, utilizing the Seahorse XF24 Extracellular Flux Analyzer (Figure 1). The Seahorse XF24 Extracellular Flux Analyzer continuously measures oxygen concentration and proton flux in the cell supernatant over time ( Wu et al., 2007 ). These measurements are converted in OCR and ECAR values and enable a direct quantification of mitochondrial respiration and glycolysis. With this protocol, we sought to assess basal mitochondrial function and mitochondrial stress of three different cancer cell lines in response to the cytotoxic test lead compound mensacarcin in order to investigate its mechanism of action. Cells were plated in XF24 cell culture plates and maintained for 24 h. Prior to analysis, the culture media was replaced with unbuffered DMEM pH 7.4 and cells were then allowed to equilibrate in a non-CO2 incubator immediately before metabolic flux analysis using the Seahorse XF to allow for precise measurements of Milli-pH unit changes. OCR and ECAR were measured under basal conditions and after injection of compounds through drug injection ports. With the described protocol we assess the basic energy metabolism profiles of the three cell lines as well as key parameters of mitochondrial function in response to our test compound and by sequential addition of mitochondria perturbing agents oligomycin, FCCP and rotenone/antimycin A. Figure 1.Overview of seahorse experiment.
Keywords: Bioenergetics; Glycolysis; Mitochondrial metabolism; Mitochondrial respiration; Seahorse XF.
Copyright © 2018 The Authors; exclusive licensee Bio-protocol LLC.
Figures


Similar articles
-
Bioenergetic characterization of mouse podocytes.Am J Physiol Cell Physiol. 2010 Aug;299(2):C464-76. doi: 10.1152/ajpcell.00563.2009. Epub 2010 May 5. Am J Physiol Cell Physiol. 2010. PMID: 20445170 Free PMC article.
-
Using Seahorse Machine to Measure OCR and ECAR in Cancer Cells.Methods Mol Biol. 2019;1928:353-363. doi: 10.1007/978-1-4939-9027-6_18. Methods Mol Biol. 2019. PMID: 30725464
-
Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity.Redox Biol. 2014 Jan 10;2:224-33. doi: 10.1016/j.redox.2013.12.028. eCollection 2014. Redox Biol. 2014. PMID: 24494197 Free PMC article.
-
From OCR and ECAR to energy: Perspectives on the design and interpretation of bioenergetics studies.J Biol Chem. 2021 Oct;297(4):101140. doi: 10.1016/j.jbc.2021.101140. Epub 2021 Aug 28. J Biol Chem. 2021. PMID: 34461088 Free PMC article. Review.
-
Alterations in bioenergetics due to changes in mitochondrial DNA copy number.Methods. 2010 Aug;51(4):452-7. doi: 10.1016/j.ymeth.2010.03.006. Epub 2010 Mar 25. Methods. 2010. PMID: 20347038 Review.
Cited by
-
Carbon Monoxide Partially Mediates Protective Effect of Resveratrol Against UVB-Induced Oxidative Stress in Human Keratinocytes.Antioxidants (Basel). 2019 Oct 1;8(10):432. doi: 10.3390/antiox8100432. Antioxidants (Basel). 2019. PMID: 31581413 Free PMC article.
-
Real-time physiological measurements of oxygen using a non-invasive self-referencing optical fiber microsensor.Nat Protoc. 2020 Feb;15(2):207-235. doi: 10.1038/s41596-019-0231-x. Epub 2020 Jan 10. Nat Protoc. 2020. PMID: 31925402 Free PMC article.
-
Keratin 17 covalently binds to alpha-enolase and exacerbates proliferation of keratinocytes in psoriasis.Int J Biol Sci. 2023 Jul 3;19(11):3395-3411. doi: 10.7150/ijbs.83141. eCollection 2023. Int J Biol Sci. 2023. PMID: 37497003 Free PMC article.
-
The Effect of Oxygen and Micronutrient Composition of Cell Growth Media on Cancer Cell Bioenergetics and Mitochondrial Networks.Biomolecules. 2021 Aug 9;11(8):1177. doi: 10.3390/biom11081177. Biomolecules. 2021. PMID: 34439843 Free PMC article.
-
Bruton Tyrosine Kinase Inhibition Decreases Inflammation and Differentially Impacts Phagocytosis and Cellular Metabolism in Mouse- and Human-derived Myeloid Cells.Immunohorizons. 2024 Sep 1;8(9):652-667. doi: 10.4049/immunohorizons.2400045. Immunohorizons. 2024. PMID: 39259208 Free PMC article.
References
-
- D’Souza G. G., Wagle M. A., Saxena V. and Shah A.(2011). Approaches for targeting mitochondria in cancer therapy. Biochim Biophys Acta 1807(6): 689-696. - PubMed
-
- Koppenol W. H., Bounds P. L. and Dang C. V.(2011). Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5): 325-337. - PubMed
-
- Serill J. D., Tan M., Fotso S., Sikorska J., Kasanah N., Hau A. M., McPhail K. L., Santosa D. A., Zabriskie T. M., Mahmud T., Viollet B., Proteau P. J. and Ishmael J. E.(2015). Apoptolodins A and C activate AMPK in metabolically sensitive cell types and are mechanistically distinct from oligomycin A. Biochem Pharmacol 93(3): 251-256. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

